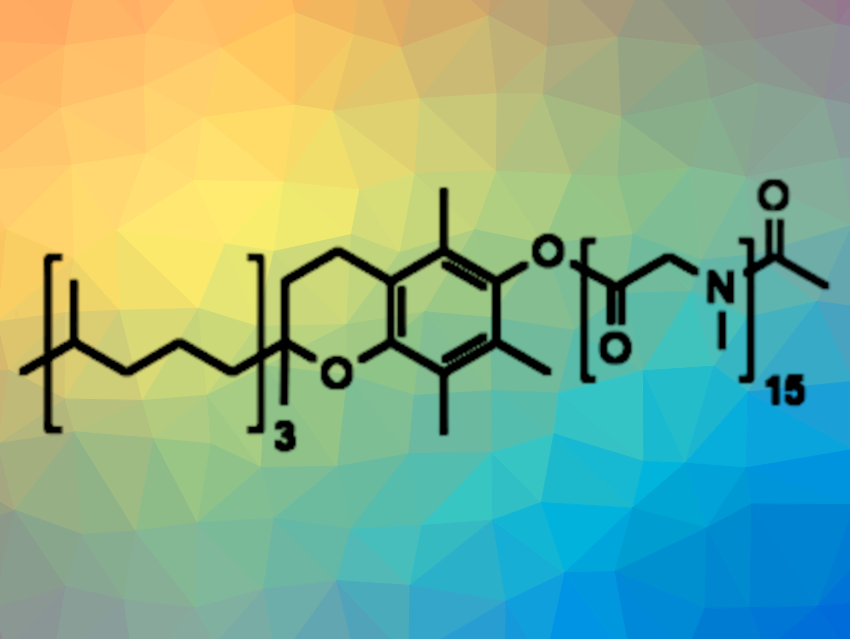
A polypeptoid made from the amino acid sarcosine can fully replace poly(ethylene glycol) (PEG) in nonionic surfactants for water-based micellar catalysis, as Bruce H. Lipshutz, University of California, Santa Barbara, USA, and colleagues have discovered [1]. Combining polysarcosine and α-tocopherol (vitamin E), the team synthesized a fully biodegradable surfactant that can support a wide range of organic transformations.
Polar, Aprotic Solvents Are “Out”, Nonionic Bioderived Surfactants Are “In”
Organic reactions require organic solvents—this concept is one of the fundamental principles in chemical synthesis. As a result, because organic transformations have such a prominent place in industrial chemistry, the chemical and pharmaceutical industries deal with huge amounts of organic solvents, most of them fossil-based—posing threats to the environment, human health, and safety alike.
To make organic chemistry both greener and safer, researchers have turned to surfactant chemistry. Above a certain concentration, known as the critical micelle concentration, surfactants self-assemble into water-soluble, nanosized spherical structures known as micelles, which have a lipophilic core and a hydrophilic surface. The lipophilic core provides a polar, aprotic nanoenvironment for organic reactions. This means that aqueous micellar catalysis, the term for reactions run in micelles in water, is a promising route for the eventual phasing out of organic solvents—or at least a drastic reduction in their use as a general reaction medium.
“Designer surfactants”, specially developed for micellar catalysis, preferably contain bioderived moieties to fulfill the promise of true green chemistry. The nonionic surfactant TPGS-750-M, developed by the Lipshutz group, for example, uses α-tocopherol (vitamin E) as the lipophilic component harboring the organic reactions [2]. As in most other designer surfactants, this lipophilic component is PEGylated to provide the ideal dispersivity and hydrophilicity in water.
The Lipshutz group found that TPGS-750-M works well for many organic reactions, but PEGylation still poses problems, especially in terms of green chemistry. As a polyether, PEG forms peroxides on prolonged exposure to air, which may complicate reactions and waste processing. In addition, it is not readily biodegradable.
Polysarcosine Replaces PEG
As a possible PEG replacement, the team chose polysarcosine, the polypeptoid of the amino acid sarcosine or N-methyl glycine. Sarcosine, a non-canonical amino acid, occurs in nature and is enzymatically degraded to glycine. For the polymerization of sarcosine, used in the form of sarcosine N-carboxyanhydride, the team used α-tocopherol as an initiator, and acetic acid anhydride to cap the N-terminus after a chain of 15 monomer units was achieved.
This simple three-step, one-pot procedure resulted in a surfactant containing polysarcosine as the hydrophilic and α-tocopherol as the lipophilic component. “Savie” (pictured above, name derived from the words sarcosine and vitamin E) appeared to be “the first designer surfactant using a polypeptoid as the hydrophilic moiety,” stated the researchers.
Savie outcompeted its PEGylated forerunner TPGS-750-M and other surfactants in terms of a number of properties. It formed more homogeneous nanosized micelles, had a lower critical micelle concentration, showed higher hydrophilicity, and could be prepared as a ready-to-use fine powder (whereas TPGS-750-M is waxy). The researchers suggest possible applications especially at high temperatures and, because of its homogeneity and salt tolerance, in microfluidics applications.
Efficient in Reactions
Savie also excelled in several important reactions in organic synthesis. The researchers tested its performance (as a 2 % aqueous solution) in a variety of industrially relevant transformations including palladium-catalyzed cross couplings, aminations, amide-bond formations, nitro group reductions, and nucleophilic organic substitutions, and found that it performed significantly better than the former TPGS-750-M standard used for comparison.
Polysarcosination rather than PEGylation may give aqueous micellar catalysis a further push toward green organic synthesis. Not only has it helped produce a fully bioderived and biodegradable surfactant, but the product also excels in terms of handling, yields, and reaction conditions.
References
[1] Joseph R. A. Kincaid, Madison J. Wong, Nnamdi Akporji, Fabrice Gallou, David M. Fialho, Bruce H. Lipshutz, Introducing Savie: A Biodegradable Surfactant Enabling Chemo and Biocatalysis and Related Reactions in Recyclable Water, J. Am. Chem. Soc. 2023, 145, 4266–4278. https://doi.org/10.1021/jacs.2c13444
[2] Bruce H. Lipshutz, Subir Ghorai, Alexander R. Abela, Ralph Moser, Takashi Nishikata, Christophe Duplais, Arkady Krasovskiy, Ricky D. Gaston, Robert C. Gadwood, TPGS-750-M: A Second-Generation Amphiphile for Metal-Catalyzed Cross-Couplings in Water at Room Temperature, J. Org. Chem. 2011, 76, 4379−4391. https://doi.org/10.1021/jo101974u