Fluorinated functional groups are useful, e.g., in pharmaceutical chemistry. Fluoralkyl units, for example, can be readily introduced into arenes, but the preparation of fluoroalkylated alkenes is less well studied. The functionalization of alkynes could be a useful approach to the synthesis of structurally complex fluoroalkylated alkenes, because it allows for the introduction of two new substituents at the same time.
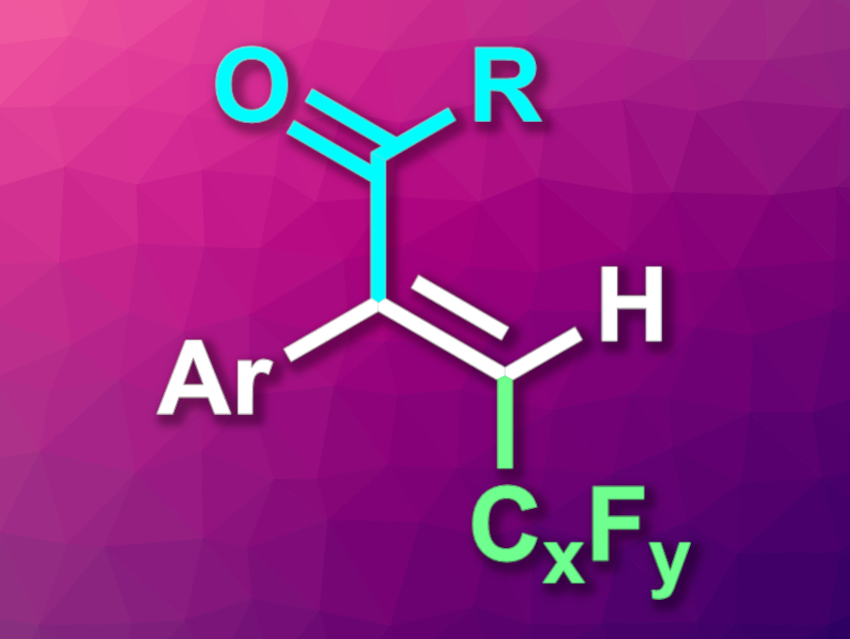
Xiao-Hong Zhang, Hai-Yong Tu, Wenzhou University, China, and colleagues have developed a nickel-catalyzed three-component reductive fluoroalkylacylation of alkynes, using fluoroalkyl halides and acyl chlorides as reactants (general product structure pictured). This reaction introduces a fluoroalkyl group (pictured in green) and an acyl unit (pictured in blue) to give fluoroalkylated enones in a regioselective and stereoselective manner.
The team used a range of aryl alkynes as substrates and reacted them with a variety of acyl chlorides and different fluoroalkyl iodides. They used NiI2 as a catalyst together with 1,10-phenanthroline as a ligand, Mn as a reductant, trimethylsilyl chloride (TMSCl) as an additive, and dimethoxyethane (DME) as the solvent. The reactions were performed at room temperature.
The desired fluoroalkylated enones were obtained in good to excellent yields and with high regio- and stereoselectivities. The reaction’s usability was demonstrated by a gram-scale example reaction that gave a yield of 85 %. The enone products can be used in further transformations. The developed approach might be useful, e.g., in medicinal chemistry and agrochemistry.
- Ni-Catalyzed Reductive Fluoroalkylacylation of Alkynes for the Steroselective Synthesis of Fluoroalkylated Enones,
Zhu-Zhu Zhang, Jia-Jia Lei, Xiao-Hong Zhang, Xing-Guo Zhang, Hai-Yong Tu,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c02464