CO2 is available in large quantities as a chemical feedstock. Due to its effects as a greenhouse gas, the conversion of CO2 to value-added chemicals is an interesting research target. One option is the synthesis of cyclic carbonates, which can be used in many applications, for example, as solvents, electrolytes, or polymer building blocks. Cyclic carbonates can be synthesized from renewable compounds with C=C double bonds, such as fatty acids or terpenes like limonene. The alkenes are first converted to the corresponding epoxides and then reacted with CO2 to form cyclic carbonates. Due to the low reactivity of CO2, suitable catalysts are necessary to promote this reaction.
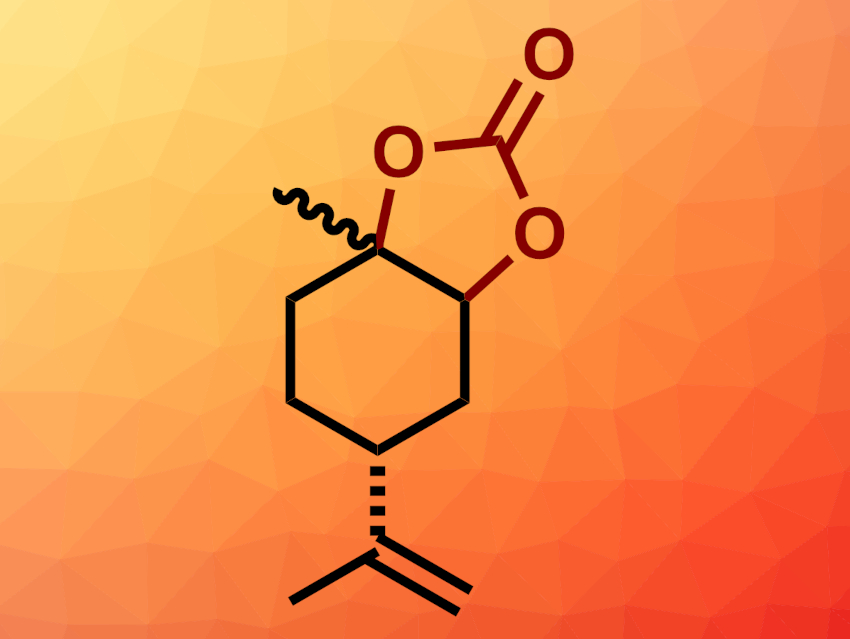
Katharina Bica-Schröder, TU Wien, Vienna, Austria, and colleagues have developed a continuous flow synthesis for limonene-based cyclic carbonates (example pictured) using supercritical CO2 as solvent and reactant with an ionic-liquid-based catalyst. Initially, the reaction was optimized under batch conditions. Different ionic liquids were tested, with tetrabutylammonium chloride (TBAC) providing the best results.
For continuous flow applications, heterogeneous catalysts are more convenient. Therefore, the team prepared a supported ionic liquid catalyst by dissolving the ionic liquid in dichloromethane with suspended silica, giving a thin film of physisorbed TBAC on silica.
In continuous-flow experiments, a limonene-monoxide-derived cyclic carbonate (pictured) was formed in 16 % overall yield under the optimized conditions, and no catalyst leaching was detected after up to 96 h. For limonene dioxide, higher overall yields of 26 % were achieved, but at the optimized loading, significant catalyst leaching was observed. Reducing the catalyst loading prevented leaching, but lowered the yields. The catalytic system has proven suitable for the selective conversion of limonene epoxides to cyclic carbonates, but further work and improved catalyst systems could lead to improved yields.
- Continuous Formation of Limonene Carbonates in Supercritical Carbon Dioxide,
Philipp Mikšovsky, Elias N. Horn, Shaghayegh Naghdi, Dominik Eder, Michael Schnürch, Katharina Bica-Schröder,
Org. Process Res. Dev. 2022.
https://doi.org/10.1021/acs.oprd.2c00143