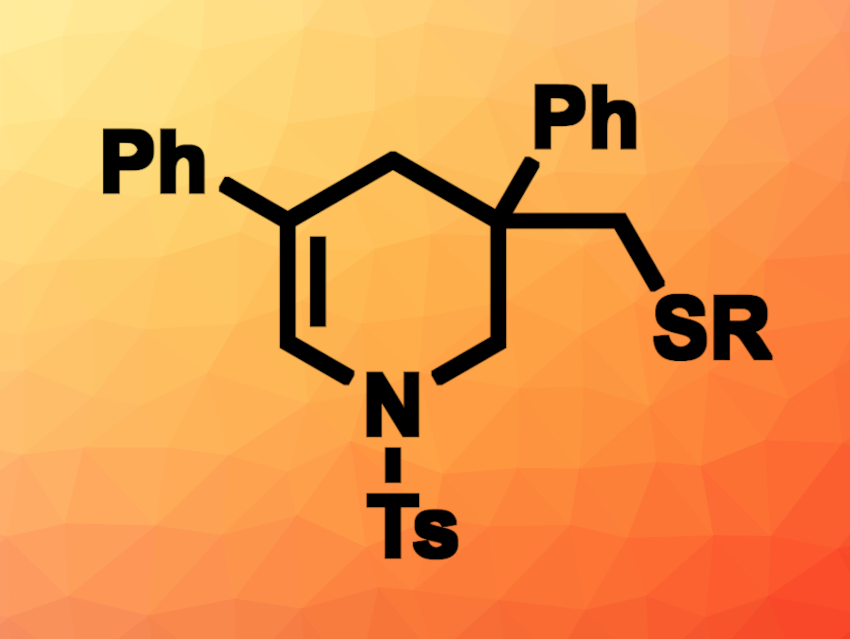
Piperidine derivatives are found, e.g., in natural products and pharmaceutically active compounds. New methods for their synthesis are interesting research targets. A carbosulfenylative functionalization of alkenes could be useful to build thiolated piperidine derivatives. However, this approach can have selectivity issues.
Pazhamalai Anbarasan, Indian Institute of Technology Madras, Chennai, India, and colleagues have developed a regioselective, acid-catalyzed intramolecular carbosulfenylation of 1,6-dienes that uses N-(aryl/alkylthio)succinimides as thiolating reagents to give dehydropiperidines (example product pictured). The team reacted 1,6-dienes such as 4-methyl-N,N-bis(2-phenylallyl)benzenesulfonamide with, e.g., N-(phenylthiol)succinimide in the presence of trifluoromethanesulfonic acid (TfOH) in dichloromethane (DCM) at room temperature and observed the desired tandem thioarylation/cyclization.
The desired thiolated dehydropiperidine derivatives were obtained in yields of up to 86 %. Benzylthiolating reagents could also be used, giving yields of up to 81 %. The researchers found that 1,6-dienes with an oxygen or CR2 linker instead of a nitrogen atom could also be used to give dihydropyran or cyclohexene derivatives, respectively.
- Acid-Promoted Carbosulfenylation of 1,6-Dienes: Selective Synthesis of Dehydropiperidines Scaffolds,
Arunachalam Kesavan, Akshaya Kumar Sahu, Pazhamalai Anbarasan,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c01274