Chiral metal-organic frameworks (MOFs) could be useful, e.g., for enantiomer separations, chiral catalysis, and sensing applications. Chiral MOFs are generally obtained from a limited pool of chiral organic precursors. Synthesizing chiral MOFs from achiral precursors could be easier and cheaper. Chirality can be transferred into MOFs, e.g., via a chiral additive or a chiral solvent environment, but this can require complicated synthetic steps. Using chiral materials such as cellulose nanocrystals (CNCs) to transfer chirality could be a promising alternative.
Vladimir V. Tsukruk, Georgia Institute of Technology, Atlanta, USA, and colleagues have developed a template-controlled synthesis of chiral MOFs from achiral precursors. The team found that chiral zeolitic imidazolate framework-8 (ZIF-8) can be grown from regular precursors via directed assembly on twisted bundles of cellulose nanocrystals. The team reacted zinc nitrate and 2-methyl imidazole, the achiral precursors of ZIF-8, in a porous, chiral nematic CNC film with a twisted organization. The CNCs were then removed from the resulting ZIF-8-CNC film using N-methyl morpholine N-oxide monohydrate (NMMO⸱H2O).
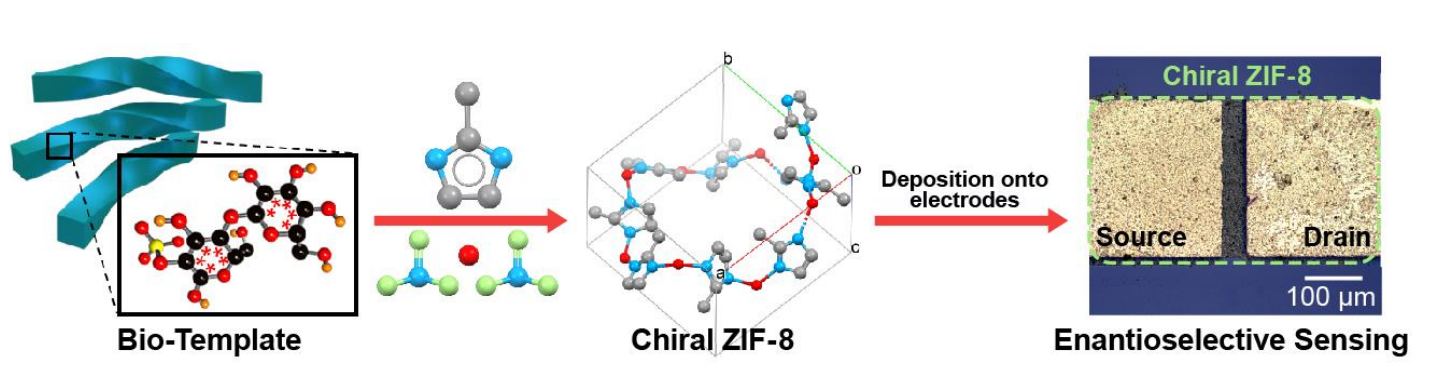
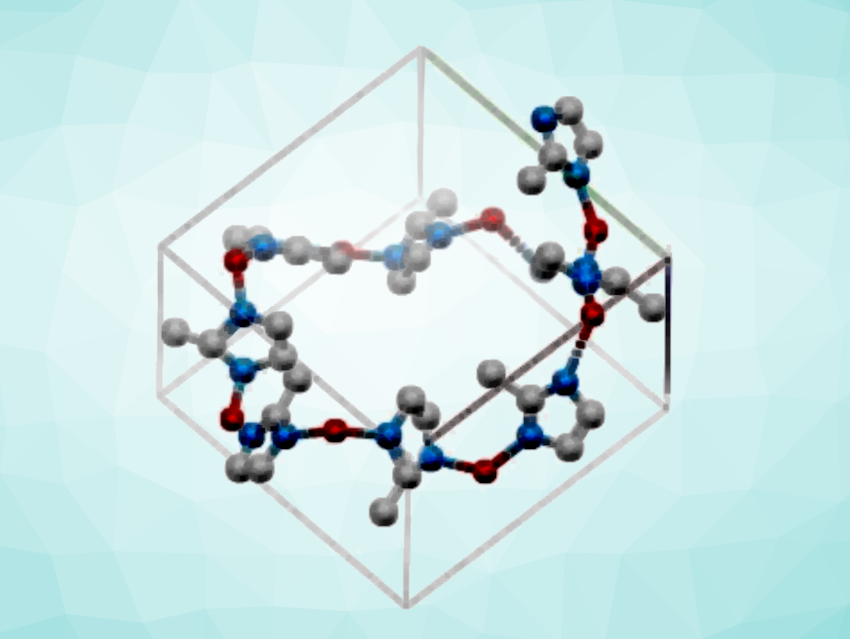
The template-grown chiral ZIF-8 has a tetragonal crystal structure (pictured) with a chiral space group of P41 instead of the cubic crystal structure of I-43m of conventional, “freely grown” ZIF-8. The team used the chiral MOF in a chemoresistive sensor to differentiate chiral amino acids in solution. The sensor based on chiral ZIF-8 showed enantioselective recognition of L– versus D-alanine with a low limit of detection. The growth of achiral precursors on twisted nano-templates might also be useful for the preparation of other chiral MOFs.
- Bio‐Templated Chiral Zeolitic Imidazolate Framework‐8 for Enantioselective Chemoresistive Sensing,
Minkyu Kim, Moon J. Han, Hansol Lee, Paraskevi Flouda, Daria Bukharina, Kellina Pierce, Katarina Adstedt, Madeline Buxton, Younghee Yoon, William T Heller, Srikanth Singamaneni, Vladimir V. Tsukruk,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202305646



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)