Here is a summary of all the questions and answers from our Art of Chemistry cover quiz. Did you get them all correct?
|
|
|
 |
The first graphic in the competition comes from an article submitted by E. A. Theodorakis, USA, in 2005 in which the first total synthesis of the natural product norrisolide was described. Norrisolide belongs to a family of diterpenes with antifungal, antimicrobial, antifeedant, antiviral and antitumor properties as well as PLA2 inhibition, but from which marine organism was it first isolated in 1983? a) marine bacterium Pseudomonas bromoutilis Answer: b) Norrisolide was first isolated from the skin extracts of the nudibranch Chromodoris norrisi. Subsequent studies led to its isolation from the marine sponges Dendrilla sp. and Dysidea sp. |
|
|
|
 |
Our next question comes from one of the front covers of this year. H. Braunschweig et al. invite us to the University of Würzburg for a showdown in the Schlenck flask between two borirene rings! Holger Braunschweig is well-known as being an expert in the chemistry of boron, but which award did he win last year for his seminal contributions to the chemistry of transition metal–boron single and multiple bonds? a) Royal Society of Chemistry Main Group Award 2014 Answer: a) Royal Society of Chemistry Main Group Award 2014 |
|
|
|
 |
In 2006 Newkome et al. described the design, synthesis, and characterization of conifer-shaped dendritic architectures. In the text accompanying the cover they quote a line from the famous poem Trees by Joyce Kilmer. The same quote appears in which film starring Gene Hackman? a) Under Fire Answer: b) Superman II |
|
|
|
 |
This cover picture comes from a Concept article by V. Balzani et al. from 2002 and uses a quote from Primo Levi’s The Periodic Table to introduce their discussion of molecular architecture in supramolecular chemistry and nanotechnology. In the article, they present a dyad structure of linked porphyrin units, containing the following elements: C, H, O, N, Zn, Fe, and Cl. Which five of these elements were used as chapter titles in The Periodic Table? a) Carbon, oxygen, zinc, iron, and chlorine Answer: c) Iron, zinc, nitrogen, hydrogen, and carbon |
|
|
|
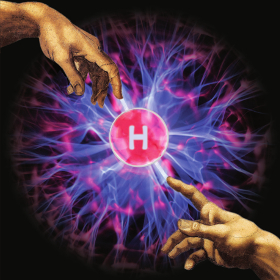 |
For their cover art of issue 2 in 2014, K. A. Jørgensen et al. use the unmistakable hands from the Creation of Adam to symbolize chirality in asymmetric synthesis. Between which years did Michael Angelo paint the ceiling of the Sistine Chapel? a) 1508-1512 Answer: a) 1508-1512 |
|
|
|
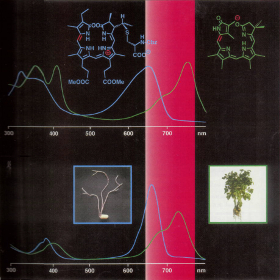 |
This cover picture was provided by K. Grubmayr et al. back in 1998 and shows the correlation of the well-known difference between plants grown in light and in darkness with UV/Vis spectra of the plant photoreceptor phytochrome (bottom), depicted alongside those of two model chromophores of known structure (top). Phytochrome is sensitive to light in the red and far-red region of the visible spectrum as shown in the cover graphic, but which of the following are photoreceptors sensitive to light in the blue region of the spectrum? a) Cryptochromes Answer: a) and c) Cryptochromes and phototropins are blue-light receptors with cryptochromes also involved in the mediation of various light-induced responses in animals. |
|
|
|
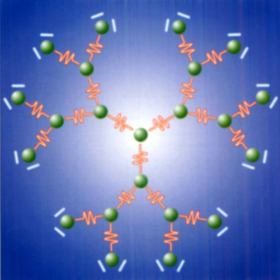 |
This question comes from an article published by V. Balzani, S. Campagna, G. Denti, et al. in 1995, in which they describe the synthesis of treelike (dendritic) structures made of RuII-polypyridine complexes. The team used a divergent procedure, based on the “complexes-as-metals and complexes-as-ligands” synthetic strategy to prepare dendrimers with 4, 10, or 22 metal ions. The dendrimer with 22 metal atoms was approximately 5 nm in size and had a molecular weight of 10890 daltons. But how many atoms did it contain in total? a) 1050 atoms Answer: c) The dendrimer contained 1090 atoms and was one of the largest Werner-type complexes prepared at the time. |
|
|
|
 |
In 2004, K. Nakanishi, K. Strømgaard, et al. used the iconic leaf of the Ginkgo biloba tree for their cover picture. The diterpene trilactones ginkgolides studied are extracted from the ginkgo leaves. Which German writer was so fascinated by the unique beauty of the Ginkgo biloba that he wrote a poem about the tree and its leaves? a) Johann Wolfgang von Goethe Answer: a) Johann Wolfgang von Goethe |
|
|
|
 |
With their cover image from 2011, L. R. Falvello, F. Palacio, et al. wanted to show us how a square net of cobalt citrate cubane single-molecule magnets are synthesized through wet chemistry. A complex based on another metal initiated the research on single-molecule magnets in the 1990’s, which metal? a) manganese Answer: a) manganese |
|
|
|
 |
This cover from 2003 illustrates a bimetallic iron complex with an unusual salt bridge, synthesized and characterized by D. B. Leznoff et al. Below the bridge the metathesis reaction, through which the bridge is formed, is shown. Yves Chauvin, Richard Schrock, and Robert Grubbs were awarded the Noble Prize in Chemistry for the development of this important reaction. In which year did these men receive their prize? a) 2004 Answer: c) 2005 |
|
|
|
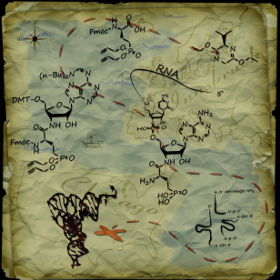 |
The cover by R. Micura, M. Simonović, et al. from 2013 shows a treasure map depicting the synthetic route to phosphonated amino acid units to the 3′-amide linked adenosine conjugates, subsequent assembly of RNA strands, and enzymatic ligation to full-length tRNASec derivatives, with the treasure marked by an X. Which children’s novel first made the idea of “X marks the spot” popular? a) Swallows and Amazons Answer: b) Treasure Island, by Robert Louis Stevenson. |
|
|
|
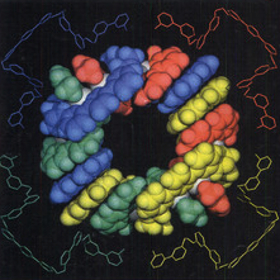 |
The journal’s first cover in 1997 came from a paper on the self-assembly of a nanocyclic CuI architecture from the group of Nobel laureate Jean-Marie Lehn. With whom did Professor Lehn share the Nobel Prize in Chemistry? a) Kenichi Fukui and Roald Hoffmann Answer: c) Jean-Marie Lehn shared the 1987 Nobel Prize in Chemistry with Donald J. Cram and Charles J. Pedersen for the development and use of molecules with structure-specific interactions of high selectivity. Fukui and Hoffmann shared the 1981 prize for their theories on the course of chemical reactions, whereas Mullis and Smith shared the 1993 prize for their work on DNA chemistry. |
|
|
|
 |
In 2012, P. Dastidar et al. created a sculpture depicting a mother and child, which was made by the group from a self-healing non-polymeric supermolecular gel. An image of this sculpture was included in their cover design. A more well-known depiction of mother and child came from the artist Damien Hirst, who won the Turner Prize for his unique work including the sculpture Mother and Child Divided, a floor-based sculpture comprising four glass-walled tanks, containing the two halves of a cow and calf, each bisected and preserved in formaldehyde solution, but in which year did he win the prize. a) 1995 Answer: a) 1995 |
|
|
|
 |
This cover from 2009 by C. Derby et al. concerns the chemistry of an L-amino acid oxidase (LAAO) enzyme in the ink secreted by the sea hare Aplysia californica as a defense against predators. LAOO enzymes function as toxins in defensive and offensive systems of a wide range of venomous creatures. Besides the sea hare, which other species employs LAOO-based toxins? a) King Cobra Answer: a) The venom of the King Cobra Ophiophagus hannah contains the L-amino acid oxidase P81383. LAOO enzymes are very common in snake venom. |
|
|
|
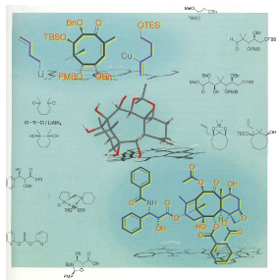 |
This week’s question come from a cover picture submitted by T. Mukaiyama et al. in 1999. It depicts structures of important starting materials and the target of a total synthesis of Taxol. Paclitaxel (Taxol) is a medication used to treat a number of types of cancer including: ovarian cancer, breast cancer, lung cancer and pancreatic cancer among others. Paclitaxel is on the World Health Organization’s List of Essential Medicines, a list of the most important medications needed in a basic health system. Which of the drugs listed below is also on that list? a) Erythromycin Answer: a) Erythromycin, an antibiotic useful for the treatment of a number of bacterial infections. It is often prescribed for people who have an allergy to penicillins. |
|
|
|
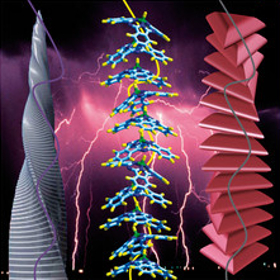 |
This cover picture from 2008 by T. Torres et al. shows that axially substituted fluorosubphthalocyanines form one-dimensional columnar assemblies in the crystalline state by spontaneous supramolecular aggregation. The structure of these assemblies resembles the helical tower “Fordham Spire” or also called “Chicago Spire”, which was planned to be the highest building in the USA. Its construction was due to be completed in 2012 but was stopped in 2008 due to financial constraints. To date the building has not been completed. Which building would it have beaten as the highest freestanding building in the USA if it had been completed on time? a) CN Tower Answer: b) Sears Tower (Willis Tower, 527 m). At its planned height of 2,000 feet (610 m), the Chicago Spire also would have been taller than the One World Trade Center, which was completed in May 2013 and is currently the tallest freestanding building in the USA (546.2 m). |
|
|
|
 |
This week’s cover picture submitted by A. Osuka et al. in 2000 shows part of a zinc/nickel windmill porphyrin array that is surrounded by different images of windmills. Windmills have served many purposes through their history from grinding grains to pumping water. In the 21st century, wind mills have taken on a new importance in the form of wind-driven turbines to generate electricity. Which country currently generates the most amount of wind energy per capita? a) Germany Answer: c) Denmark. Denmark generates over 30 % of electricity from wind power and aims to get 50 % of its electricity from wind power by 2020. Spain, Portugal, Sweden, and Germany are the following top wind power countries per capita. |
|
|
|
 |
This week’s cover question comes from an article published in 2010 by J. Ryan et al. It depicts a Colombian poison arrow frog, Dendrobates histrionicus, which naturally produces a histrionicotoxin spiropiperidine alkaloid, and the paper describes a synthetic method for an unnatural analogue of this biologically valuable compound. Which album, released in 1984, features a song called ‘Poison Arrows’? a) The Smiths by The Smiths Answer: b) Discovery by Mike Oldfield |
|
|
|
 |
In 2007, M. Sollogoub et al. included a picture of the Moct (Palace Bridge), a famous bascule bridge, in their cover image. The bridge was used to illustrate their concept of a bascule-bridge strategy for the clockwise deprotection of a reversibly capped cyclodextrin. The Moct was opened in 1916 and was designed by Andrey Pshensitsky, but in which city does it reside? a) Minsk Answer: c) St. Petersburg |
|
|
|
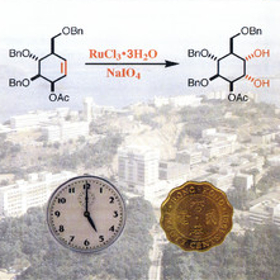 |
Our final question is based on a cover from T. K. M. Shing et al. published in 1996. The cover shows the syn-dihydroxylation of an alkene by oxidative ruthenium catalysis. The clock represents the short amount of time it takes for the reaction to go to completion and the coin the low cost of ruthenium compounds compared to osmium tetroxide, which is the standard reagent for dihydroxylation. The coin is Hong Kong currency as this is where the authors were based. Prior to the establishment of the Special Administrative Regions (SAR), coins with Queen Elizabeth II’s portrait have been gradually withdrawn from circulation. Which national symbol can now be found on many of Hong Kong’s coins? a) Hibiscus rosa-sinensis Answer: b) Bauhinia flower. Hibiscus rosa-sinensis is a national symbol of Malaysia and cherry blossom is a national symbol of Japan. |
|
|
|