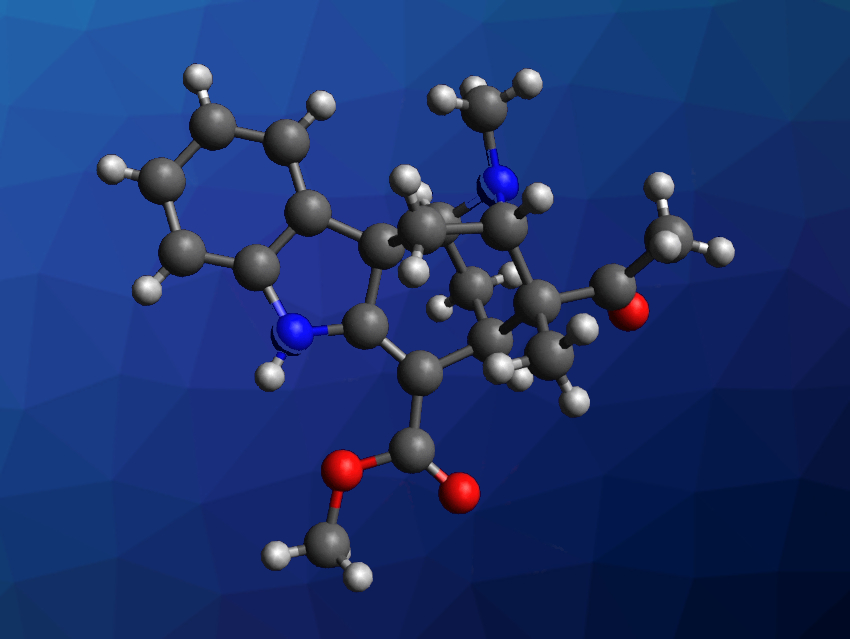
Monoterpenoid indole alkaloids often have interesting structures and biological activities. Akuammiline alkaloids, for example, can be isolated from Alstonia scholaris, or blackboard trees, and often feature cage-like structures that are challenging targets for chemical synthesis. (+)-Alstonlarsine A (pictured) is one such alkaloid that was isolated from the roots and barks of Alstonia scholaris. The compound features a piperidine- and pyrrolidine-bridged cyclohepta[b]indole core and five stereogenic carbon centers. It has shown some biological activity in preliminary studies, but the available amounts of (+)-alstonlarsine A were not sufficient for in-depth studies.
Hongbin Zhai, Shenzhen Graduate School of Peking University, Shenzhen Bay Laboratory, and Shenzhen Polytechnic, China, and Collaborative Innovation Center of Chemical Science and Engineering, Tianjin, China, and colleagues have performed the first asymmetric total synthesis of (+)-alstonlarsine A. The team started from tryptophol, a functionalized indole, and 2-methyl-2-cyclopenten-1-one. These two compounds were functionalized to give two building blocks, a 2-indolyl-substituted dimethyl malonate and a 2-methyl-2-cyclopentenyl carbonate. These building blocks were then connected in an asymmetric allylic alkylation reaction.
The resulting intermediate was subjected to an intramolecular [3+2] cycloaddition to construct the cyclohepta[b]indole framework. Further functionalizations and an interrupted Pictet–Spengler reaction, which was used to build the piperidine- and pyrrolidine-bridged framework, then gave the desired (+)-alstonlarsine A.
The product was obtained in 13 steps from the malonate and carbonate building blocks. According to the researchers, the strategy could also be useful for the synthesis of other related cyclohepta[b]indole alkaloids.
- Asymmetric Total Synthesis of (+)-Alstonlarsine A,
Jun-Jun Yao, Rui Ding, Xiaoming Chen, Hongbin Zhai,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c06518