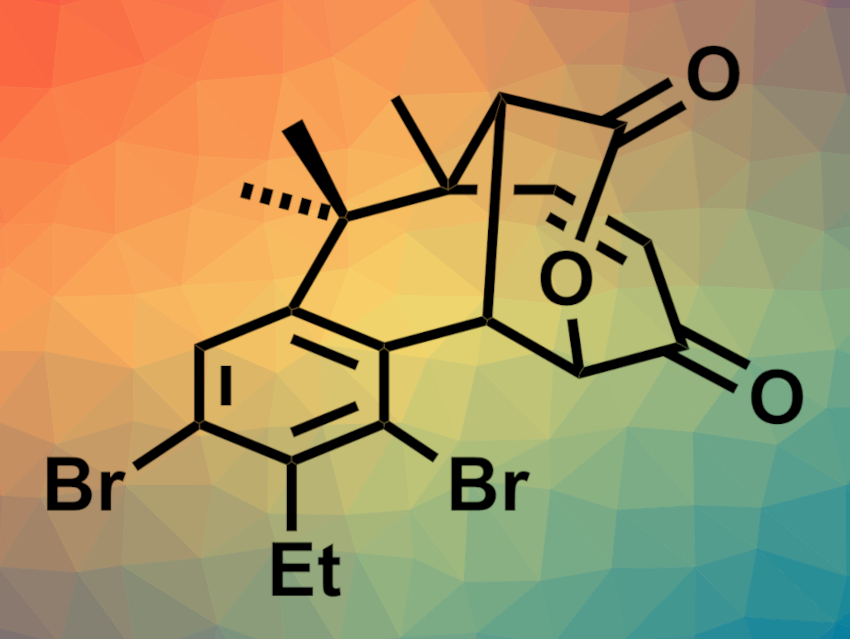
Salimabromide (pictured) is a natural product that was isolated from marine myxobacteria. It has shown interesting bioactivity in preliminary studies, but it is only available in very small amounts in Nature, which has hampered further investigations. Salimabromide has a benzo-fused [4.3.1] carbon skeleton with four contiguous stereocenters. Its complex structure makes it a challenge for organic synthesis.
Hai-Hua Lu, Westlake University and Westlake Institute for Advanced Study, Hangzhou, China, and colleagues have performed a concise total synthesis of salimabromide. The team started from cycloheptadienone, which was subjected to a tandem Michael/Mukaiyama aldol reaction to attach an aromatic fragment. The addition of methyl lithium and an oxidative rearrangement then led to a precursor for the key step—an intramolecular radical cyclization mediated by hydrogen atom transfer (HAT). This cyclization builds the benzo-fused [4.3.1] carbon skeleton.
Subsequent dehydrogenation, reduction, and dehydration gave a diene, which was then used to form the lactone ring of salimabromide via a tandem oxidative cyclization. Finally, a dibromination of the arene gave the desired salimabromide. The team also performed an enantioselective synthesis of (+)-salimabromide, with the enantioselective hydrogenation of a cycloheptenone intermediate as a key step. According to the researchers, the transformations used in this synthesis could also have other applications in organic synthesis.
- Concise Total Synthesis of Salimabromide,
Hai-Hua Lu, Kang-Ji Gan, Fu-Qiang Ni, Zhihan Zhang, Yao Zhu,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c08337