Compounds with multiple bonds between the heavier main group elements are interesting research targets in inorganic chemistry. The isolation of such species can be challenging due to the reduced tendency to form multiple bonds compared with carbon and the resulting higher reactivity, e.g., towards oligomerization. Stabilization using sterically demanding substituents or via the formation of Lewis-acid or base adducts can be useful in this context.
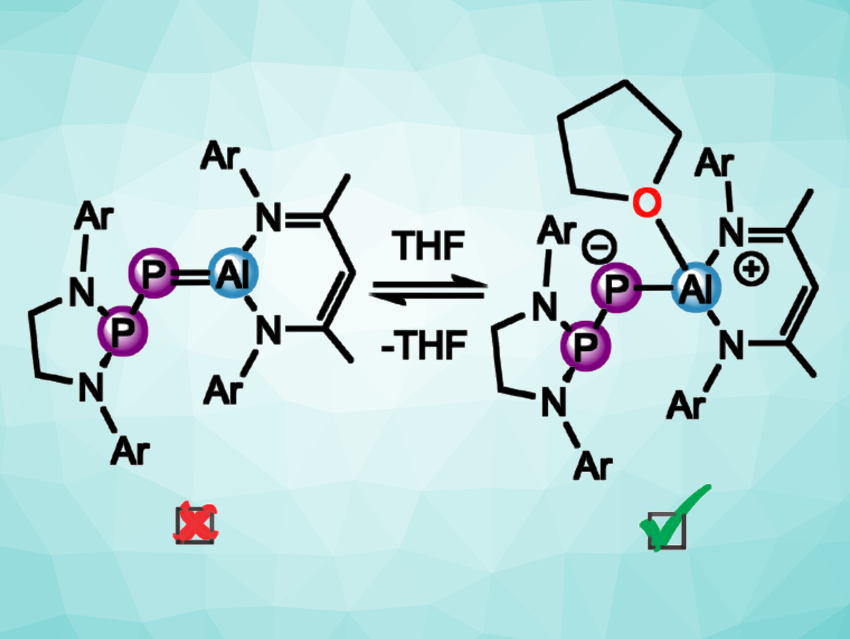
Jose M. Goicoechea, Indiana University, Bloomington, USA, and colleagues have attempted to isolate a compound with an Al=P double bond connected to another P atom, a phosphanyl-phosphaalumene (pictured above on the left, Ar = Dipp, or 2,6- iPr2C6H3). The team performed a reaction of Al(NacNac) (NacNac = HC[C(Me)N(Dipp)]2) with [H2CN(Dipp)]2PPCO, but they observed that the target product was only formed as a transient species at –70 °C and rapidly underwent an intramolecular C–H activation at the NacNac ligand when warmed to room temperature. This resulted in the formation of an undesired C–Al bond.
The team studied the reactivity of the transient phosphanyl-phosphaalumene species at low temperatures and found that it can activate small molecules such as H2 and CO2, as well as N–H bonds and Si–H bonds in amines and silanes, for example. To enable the isolation of the elusive target species, the team used tetrahydrofuran (THF) as a Lewis base. After recrystallization, they obtained the desired THF adduct (pictured above on the right) in the form of reddish-purple, needle-shaped crystals that were suitable for single-crystal X-ray diffraction. The adduct is stable for hours at room temperature and for days at –30 °C. The researchers found that the THF adduct retains at least some of the reactivity of the transient P=Al-type species.
- Trapping an Elusive Phosphanyl‐Phosphaalumene,
Lilian S. Szych, Lars Denker, Joey Feld, Jose Goicoechea,
Chem. Eur. J. 2024.
https://doi.org/10.1002/chem.202401326