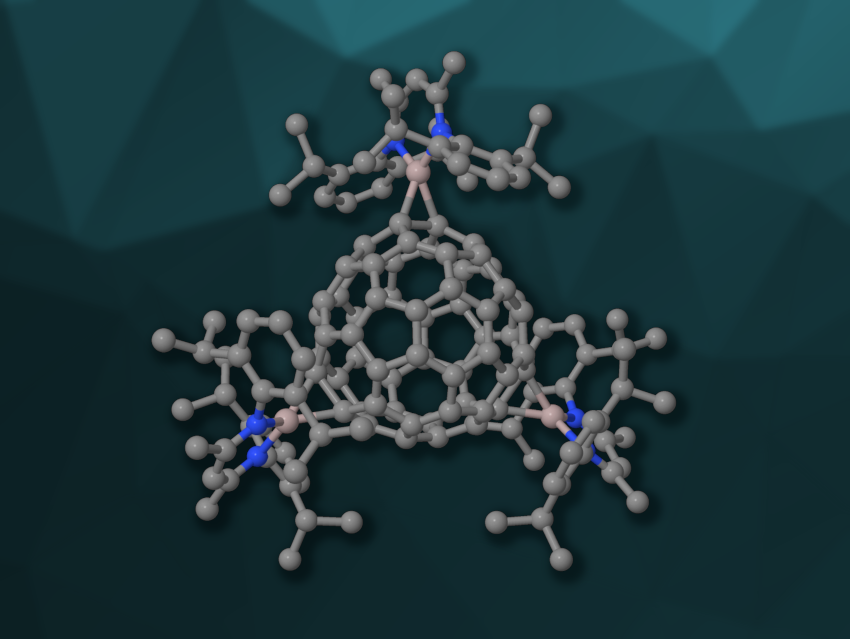
The buckminsterfullerene C60 can form fullerides, i.e., compounds containing fullerene anions. The intercalation of alkali or alkaline-earth metals (A) into the lattice of C60 gives compounds of the type AnC60. Well-defined ionic fulleride complexes can also be prepared, e.g., via the reduction of C60 with magnesium(I) reagents. Less is known about compounds that contain aluminium and fullerenes.
Andreas Stasch, University of St Andrews, UK, Robert Kretschmer, Chemnitz University of Technology, Germany, and colleagues have prepared the first example of a structurally characterized aluminium-fulleride complex, i.e., [{(Dippnacnac)Al}3C60] (simplified structure pictured; Dippnacnac = HC{CH3CN(Dipp)}2, Dipp = 2,6-iPr2C6H3). The team reacted three equivalents of the alumylene [(Dippnacnac)Al] with C60 in benzene or toluene at room temperature. The aluminium-fulleride complex was obtained in the form of deep red single crystals.
The product was characterized using X-ray diffraction, 1H and 13C NMR spectroscopy, and UV/Vis spectroscopy, and its electronic structure was investigated using density functional theory (DFT) calculations. The researchers found that the complex crystallizes in the space group C2/c. The bound aluminium atoms form AlC2 metallacyclopropane-type groups at the bonds fusing two six-membered rings. The team investigated whether the aluminium-fulleride complex can be used for further transformations. They found that the reaction with the magnesium(I) complex [{(Mesnacnac)Mg}2] (Mes = 2,4,6-Me3C6H2) removed all [(Dippnacnac)Al] groups and produced the fulleride [{(Mesnacnac)Mg}6C60].
- A molecular aluminium fulleride,
Samuel Ray Lawrence, Tobias Rüffer, Andreas Stasch, Robert Kretschmer,
Chem. Commun. 2023.
https://doi.org/10.1039/D3CC01609A