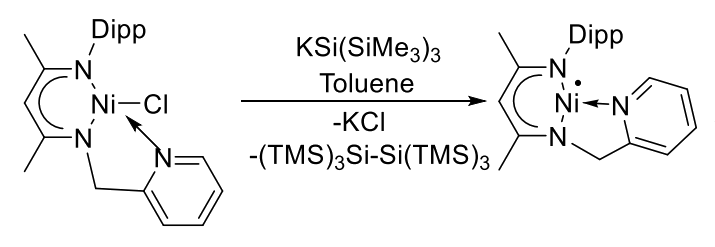
Sakya S. Sen, CSIR-National Chemical Laboratory, Pune, India, and Academy of Scientific and Innovative Research (AcSIR), Ghaziabad, India, and colleagues have synthesized a nickel(I) radical with a T-shaped structure. The team used KSi(SiMe3)3 as a reducing agent to convert a Ni(II) chloride complex with a picolyl-group-containing β-diketiminato ligand, i.e., [2,6-iPr2-C6H3NC(Me)CHC(Me)NH(CH2py)] Ni(II)Cl, into the desired Ni(I) radical (pictured below).
The formation of the Ni(I) radical was confirmed using electron paramagnetic resonance (EPR) measurements and a reaction with the radical scavenger TEMPO, resulting in the formation of an unusual three-membered nickeloxaziridine complex.
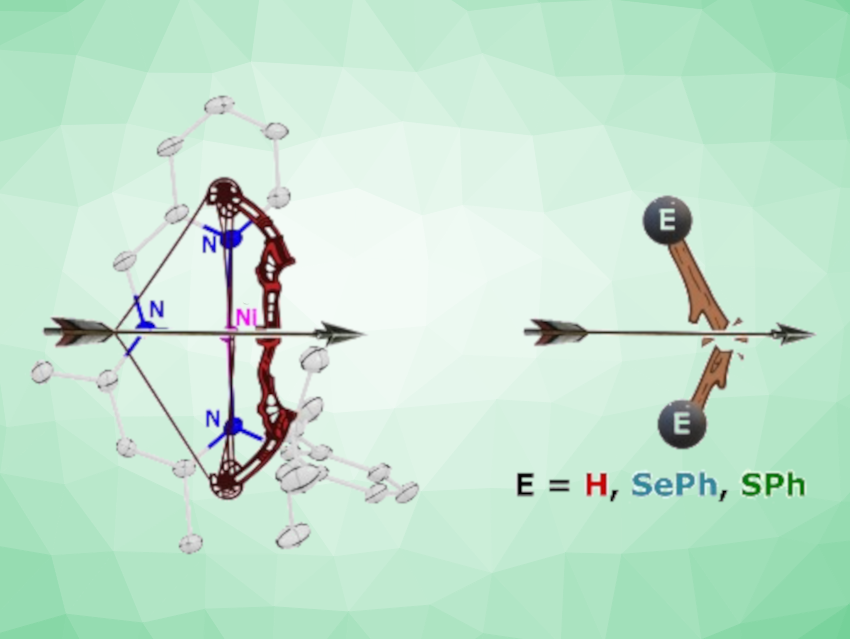
The researchers tested the radical’s activity for dihydrogen activation, resulting in the formation of a monomeric Ni hydride under ambient reaction conditions. The Ni(I) radical also homolytically cleaved the S‒S and Se‒Se bonds of a disulfide and a diselenide. This led to interesting C–C coupled products in which two units of the Ni complex are connected via their ligands.
Overall, the work provides a unique approach to synthesizing and stabilizing Ni(I) radicals. This approach could be useful in organic synthesis and catalysis.
- Tridentate NacNac Tames T‐Shaped Nickel(I) Radical,
Sanjukta Pahar, Vishal Sharma, K. Vipin Raj, Mayur P. Sangole, Christy P. George, Kirandeep Singh, Kumar Vanka, Rajesh G. Gonnade, Sakya S. Sen,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202303957