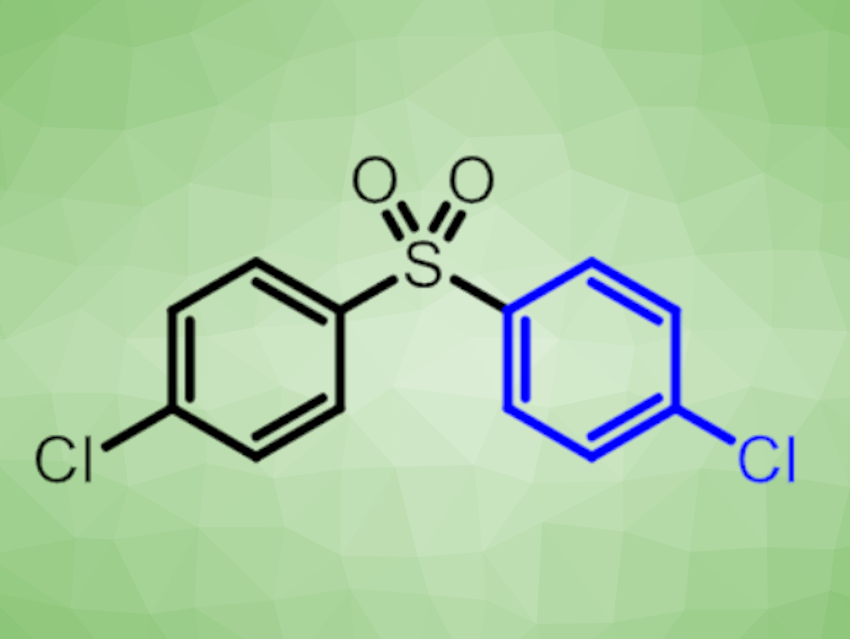
High-performance thermoplastics are low-volume, expensive polymers designed to withstand high temperatures. One of the most used high-performance thermoplastics is polyether sulfone (PES) (pictured below). One route for the synthesis of PES is the polycondensation reaction of 4,4′-dichlorodiphenyl sulfone (4,4′-DCDPS, see below) and a diphenoxide. However, the commonly used synthetic routes to 4,4′-DCDPS produce large amounts of stoichiometric by-products.
Thomas Schaub, Catalysis Research Laboratory (CaRLa), Heidelberg, Germany, and BASF SE, Ludwigshafen, Germany, and colleagues have developed a metal-free Friedel-Crafts sulfonylation for the synthesis of diaryl sulfones such as 4,4′-DCDPS (pictured below in the green box). The team used triflic acid (TfOH) as an acidic catalyst for the neat reaction of arylsulfonyl chlorides with arenes at 160 °C.
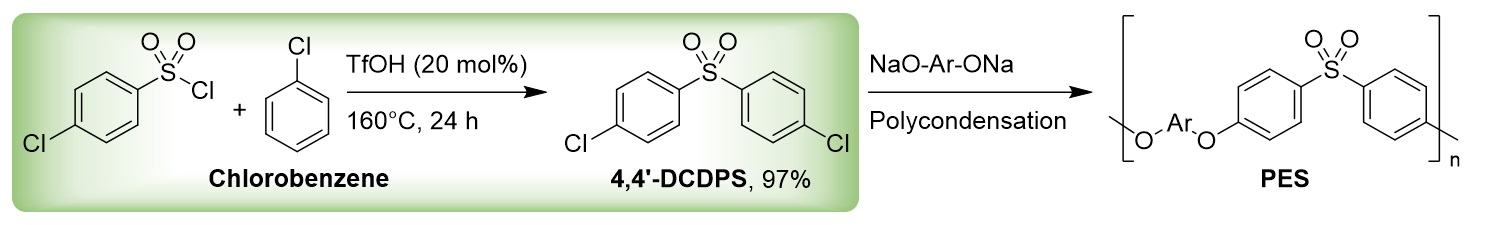
The reaction gives a range of different sulfone derivatives with high selectivity and in good to excellent yields. This approach offers a straightforward route to 4,4′-DCDPS. The catalyst and the excess arene can be easily recovered and reused in further batches.
- Triflic Acid‐Catalyzed Friedel‐Crafts Reaction for the Synthesis of Diaryl Sulfones,
Alban Falconnet, Jan-Dirk Arndt, A. Stephen K. Hashmi, Thomas Schaub,
Eur. J. Org. Chem. 2022.
https://doi.org/10.1002/ejoc.202200477