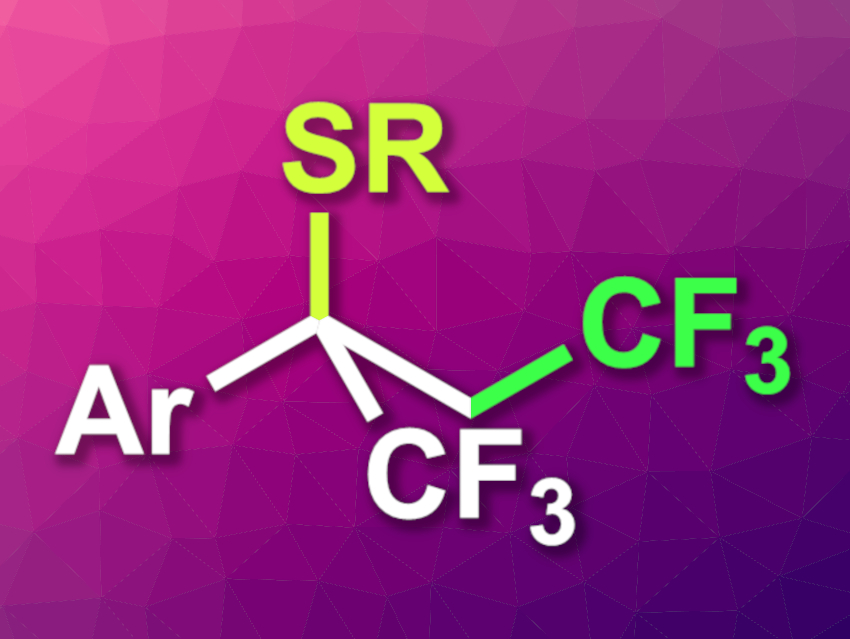
Trifluoromethyl groups can be useful, e.g., in pharmaceutical chemistry, where they can help to tune the properties of drug candidates. The synthesis of trifluoromethylated compounds has been well-explored, while methods for the preparation of 1,2-bis(trifluoromethylated) derivatives have received less research attention. The compound α-(trifluoromethyl)styrene is a promising starting point for such syntheses. However, it can be challenging to avoid a defluorination of the existing trifluoromethyl group when this substrate is used.
Xiaonan Zhang, Anhui Agricultural University, Hefei, China, and Yahui Li, Anhui Agricultural University and Guizhou University, Guiyang, China, have developed a photoinduced, iron-catalyzed thiotrifluoromethylation of α-(trifluoromethyl)styrene (general product structure pictured) or other aryl alkenes that proceeds without defluorination. The team reacted the substrates with different thiophenol derivatives and CF2SO2Cl, using NEt3 as a base, tris(acetylacetonato) iron(III) (Fe(acac)3) as a catalyst precursor, and ethyl acetate as the solvent. The reactions were performed under blue LED light at room temperature.
The desired 1,2-bis(trifluoromethylated) products—or trifluoromethylated products for simple aryl alkene substrates—were obtained in moderate to good yields. The reaction has a broad substrate scope and can be used as a flow reaction to obtain products on a gram scale. The researchers propose a radical mechanism for the transformation.
- Light-Induced Iron-Catalyzed Trifluoromethylative Thiolation of Alkenes,
Xiaonan Zhang, Yahui Li,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c03348