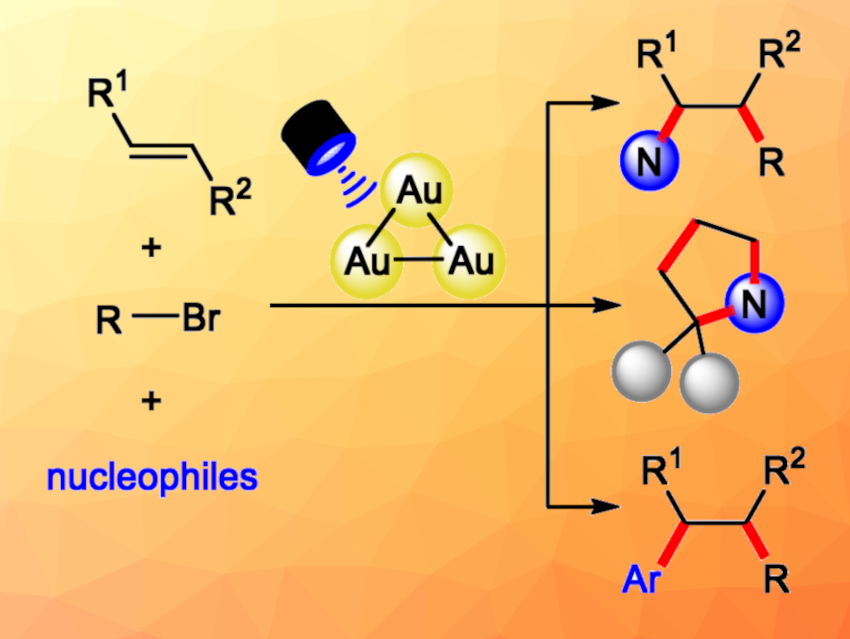
Ruthenium-and iridium-based complexes for photoredox catalysis are useful tools in organic synthesis. Together with such photocatalysts, activated alkyl halides have been used to generate alkyl radicals for the 1,2-difunctionalization of alkenes. However, unactivated alkyl bromides are challenging substrates for this type of reaction due to their redox potentials. Catalysts based on multinuclear gold complexes could help to overcome these redox potential limitations of alkyl bromides.
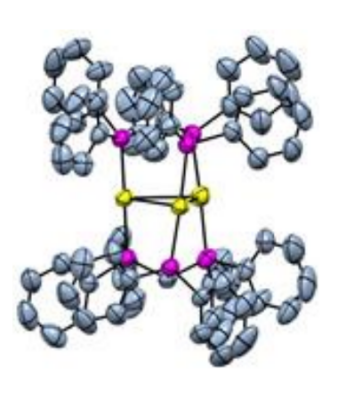
Jin Xie, Nanjing University, China, and Xinjiang University, Urumqi, China, and colleagues have developed a method for a three-component 1,2-difunctionalization of alkenes with unactivated alkyl bromides and nucleophiles such as amines and indoles (general reactions pictured above), using the trinuclear gold catalyst [Au3(tppm)2](OTf)3 (pictured below, tppm = 1,1,1-tris(diphenylphosphino)methane). This protocol can be used for 1,2-aminoalkylations and 1,2-alkylarylations of styrenes.
The team used NaOAc as a base and MeCN as the solvent, and the reactions were performed under blue LED light at room temperature. The desired difunctionalized products were obtained in moderate to high yields (up to 96 %). The reaction has a broad reaction scope and good functional group tolerance (over 100 examples). It can also be used for a formal [2+2+1] cyclization to construct pyrrolidine derivatives. For this, a 1,2-aminoalkylation reaction with 1-bromo-2-chloroethane is performed, which is followed by an intramolecular SN2 cyclization.
- Trinuclear Gold‐Catalyzed 1,2‐Difunctionalization of Alkenes,
Qing-Yun Fang, Jie Han, Mingzhe Qin, Weipeng Li, Chengjian Zhu, Jin Xie,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202305121