The increasing concentration of CO2 in the atmosphere strongly contributes to climate change. In addition to the reduction of CO2 emissions, the removal of CO2 from the atmosphere could help to tackle this global issue. The sustainability of CO2 removal improves significantly if the CO2 captured from air is converted to value-added products instead of just being stored. This process is called direct air capture and conversion (DACC). Most CO2 reduction catalysts require pure CO2 as a feed gas and cannot work with the relatively small amount of CO2 in the air. The presence of oxygen poses an additional challenge for the activity of the catalytic centers.
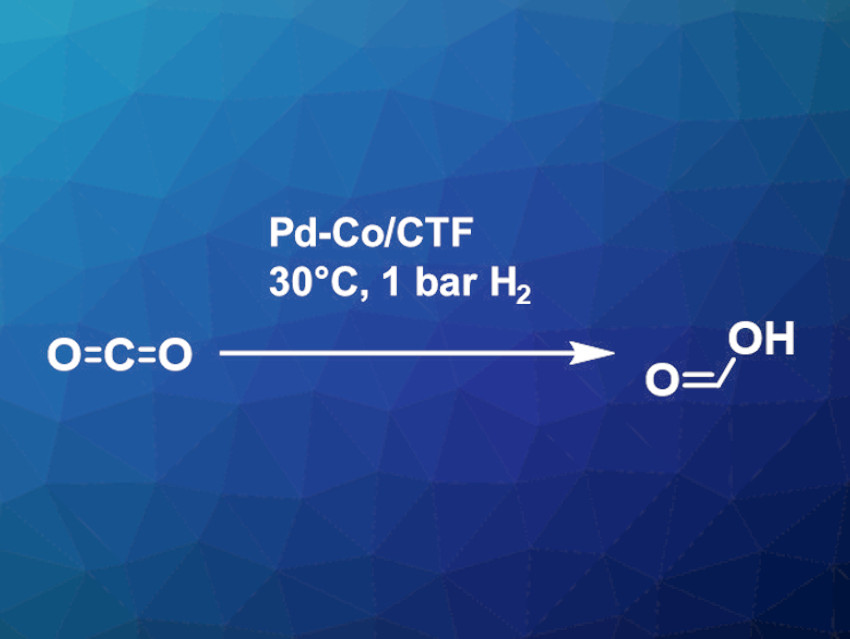
Guoqing Ren, Weiqiao Deng, Shandong University, Qingdao, China, and colleagues have developed a heteronuclear dual single-atom catalyst for the conversion of CO2 to formate under ambient conditions. The catalyst contains palladium and cobalt atoms supported on a covalent triazine framework (CTF). CO2 is captured from the air by an aqueous solution of triethylamine, in which bicarbonate is formed. The catalyst converts this to formate, which can serve as a feedstock for further conversions or as a liquid hydrogen storage material.
The team synthesized the CTF from 2,6-pyridinedicarbonitrile, which was heated with zinc chloride under vacuum. After removal of ZnCl2, the desired palladium and cobalt-functionalized material was formed by the reaction of metal precursors (palladium(II) acetylacetonate and cobalt(III) acetylacetonate) with the CTF in ethanol at 50 °C over 12 h. The catalyst was characterized by Fourier-transform infrared (FT-IR) spectroscopy, X-ray absorption spectroscopy, and different microscopy techniques.
The capture and hydrogenation of CO2 were carried out at 30 °C under 1 bar of hydrogen. The optimized catalyst exhibits a high TON of 59.6 under ambient conditions. It can be used to directly hydrogenate the CO2 captured from air into formate with a conversion of up to 84.6 %. The catalyst was successfully recycled four times. The addition of cobalt improved the turnover number by a factor of four compared to using only palladium. The cobalt binds and activates the CO2, while the palladium activates the hydrogen.
- Heteronuclear Dual Single-Atom Catalysts for Ambient Conversion of CO2 from Air to Formate,
Shengliang Zhai, Jikai Sun, Lei Sun, Li Yang, Rui Tu, Shuchao Jiang, Tie Yu, Hao Wu, Chengcheng Liu, Zhen Li, Dong Zhai, Youyong Li, Guoqing Ren, Weiqiao Deng,
ACS Catal. 2023.
https://doi.org/10.1021/acscatal.2c06033