Peptides can be powerful biological agents, but they are quickly broken down in the body. Dilution, internalization, and enzymatic degradation mean it is challenging to deliver them unharmed and in concentrated form to their target sites. Yongju Kim, Korea University, Seoul, Republic of Korea, and colleagues have developed a supramolecular method to assemble and concentrate active peptide ligands in a two-dimensional array, which spreads out into a large, flat sheet. This sheet could activate cell membrane receptors much more efficiently than any other peptide delivery form [1].
Cell Membrane Receptors Bind Peptides
Many receptor proteins in nature exist in two-dimensional cell membranes. After binding their antigens, they pass a signal to the cell interior to trigger a biological response. Most antigens are whole proteins, but the motif in the protein which binds to the receptor may just be a small part of the protein, a short amino acid sequence with a characteristic shape. As peptides are easy to synthesize and easier to handle than whole proteins, medicinal chemists prefer them as potential drug candidates.
However, native peptides are usually short-lived in the body. They are diluted and enzymatically degraded before they can reach their targets. One method for protecting and concentrating peptides before they reach their targets is wrapping them up or coupling them to nanocarrier structures. However, this is complicated and requires at least a release mechanism to expose the ligands once they are at their target site. Instead of using three-dimensional packages, the team turned to making sheets, embedding the peptides in a large and well-defined two-dimensional array.
Self-Assembly of Amphiphilic Peptides
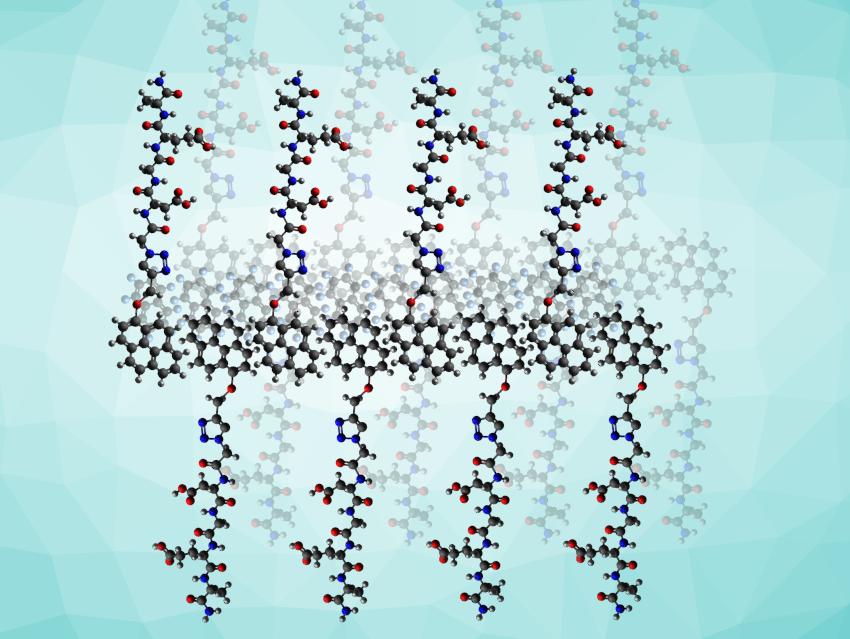
The team chose the two-dimensional form because a large flat sheet will cover part of the cell surface, making it harder for the cell to internalize single peptides. The supramolecular arrangement also protects the peptides, says Kim, “The peptide assembly, which is highly integrated, showed resistance to enzymatic degradation. This means that the two-dimensional peptide material can stably and continuously interact with membrane proteins outside the cell membrane.”
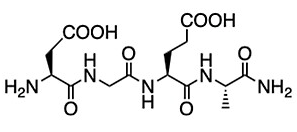
As the peptide of interest, the team investigated a strand containing the four amino acids asparagine, glycine, glutamine, and alanine (DGEA, pictured below), a sequence derived from collagen type I proteins and known to bind the integrin α2β1 receptor.
Integrins are membrane proteins that regulate cellular adhesion and play a vital role in signal transduction. Their activation by proteins of the extracellular matrix such as collagen promotes cell proliferation and wound healing.
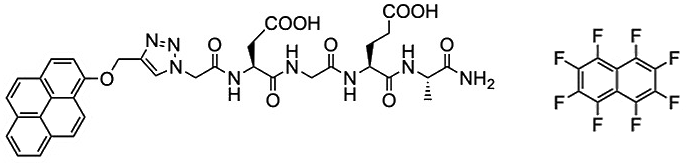
To prepare the DGEA peptide for self-assembly, the team grafted its asparagine terminus to pyrene, a polycyclic aromatic hydrocarbon with four fused benzene rings (product pictured below on the left). Drawn together by the aromatic interactions of the pyrenes, this amphiphilic grafted DGEA peptide self-assembled to form vesicular structures of almost half a micron in diameter. The addition of octafluoronaphthalene (pictured below on the right) broke the vesicles down and established the lateral sheets, having the peptides well exposed on both sides of the sheet.
Arene–Perfluoroarene Interactions
This was made possible because the octafluoronaphtalene molecule promotes stacking with the pyrene molecule. This aromatic interaction, which is extraordinarily stable in solution, is known as arene–perfluoroarene interaction, and is considered to be unproblematic for biological systems [2]. “The reason for using the arene-perfluoroarene interaction is that this interaction is reported to be biorthogonal,” says Kim. This means the reaction has no additional impact on the cell beyond the desired therapeutic response.
The team found that the peptide ligand sheets enhanced cell proliferation and differentiation when applied to myoblast cells (muscle cell precursors). Since no vesicles, native peptides, or other aggregation forms showed this response, the researchers concluded that only the sheets provided the peptide in a concentration that activated the receptors.
Two-dimensional supramolecular peptide arrays are an unprecedented delivery form for medical or biological purposes. To date, micelles and fibers have been the usual options for concentrating and protecting peptides. However, the team demonstrated that large, flat shapes could afford huge advantages for interactions with membranes and membrane receptors.
References
[1] Taeyeon Kim, Jinwoo Hong, Jehan Kim, Jinhan Cho, and Yongju Kim, Two-Dimensional Peptide Assembly via Arene−Perfluoroarene Interactions for Proliferation and Differentiation of Myoblasts, J. Am. Chem. Soc. 2023, 145, 1793−1802. https://doi.org/10.1021/jacs.2c10938
[2] Ga Young Lee, Elizabeth Hu, Arnold L. Rheingold, K. N. Houk, Ellen M. Sletten, Arene-Perfluoroarene Interactions in Solution, J. Org. Chem. 2021, 86, 8425–8436. https://doi.org/10.1021/acs.joc.1c00921