Lithium salts make batteries powerful but expensive. An ultralow-concentration electrolyte based on the lithium salt LiDFOB may be a more economical and more sustainable alternative. Jinliang Yuan, Lan Xia, Ningbo University, Zhejiang, China, Xianyong Wu, University of Puerto Rico-Rio Piedras Campus, San Juan, USA, and colleagues have demonstrated that cells using these electrolytes and conventional electrodes have high performance. In addition, the electrolyte could facilitate both production and recycling of the batteries.
Expensive Electrolytes
Lithium-ion batteries (LIBs) provide power to smartphones and tablets, drive electric vehicles, and store electricity at power plants. The main components of most LIBs are lithium cobalt oxide (LCO) cathodes, graphite anodes, and liquid electrolytes that deliver mobile ions for the decoupled cathode and anode reactions. These electrolytes determine the properties of the interphase layer that forms on the electrodes and thus affect features such as battery cycling performance.
However, commercial electrolytes are still mostly based on a system formulated over 30 years ago: 1.0 to 1.2 mol/L lithium hexafluorophosphate (LiPF6) in carboxylic acid esters (“carbonate solvent”). Over the last ten years, high-concentration electrolytes (> 3 mol/L) have been developed, increasing battery performance by favoring the formation of robust inorganic-dominated interphase layers.
However, these electrolytes have high viscosity, poor wetting ability, and inferior conductivity. The large amounts of lithium salts required also make them very expensive, often a critical parameter for feasibility. To reduce costs, research has also begun into ultralow-concentration electrolytes (< 0.3 mol/L). The drawback for these has been that the battery cell decomposes more solvent than the few salt anions, which leads to an organic-dominated and less stable interphase layer.
A Practical Alternative
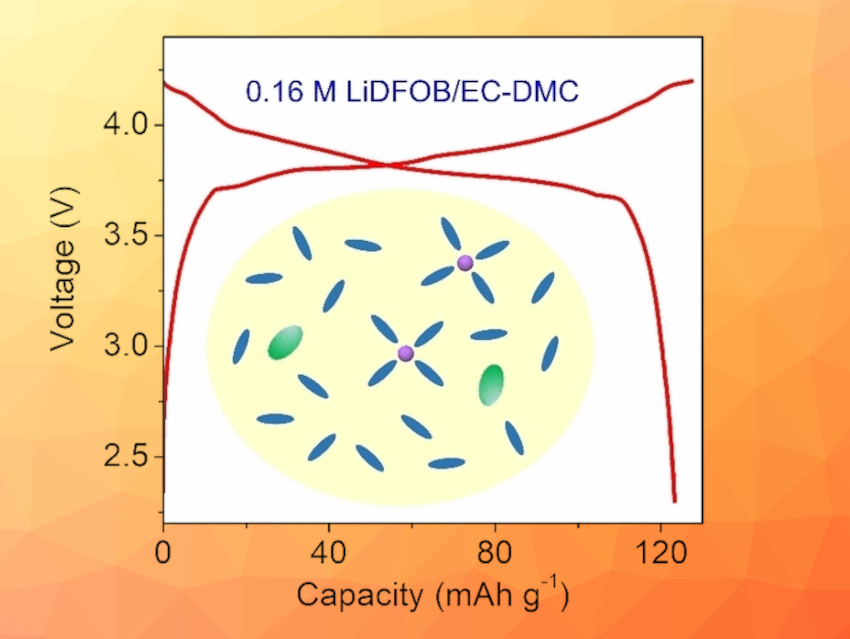
The team developed an ultralow-concentration electrolyte that may be suitable for practical application in lithium-ion batteries: LiDFOB/EC-DMC. LiDFOB (lithium difluoro(oxalato)borate) is a common additive and significantly cheaper than LiPF6. EC-DMC (ethyl carbonate/dimethyl carbonate) is a commercial carbonate solvent.
The electrolyte has a potentially record-breaking low salt content of 2 weight percent (0.16 mol/L) but a sufficiently high ionic conductivity (4.6 mS/cm) to operate a battery. In addition, the properties of the DFOB– anions allow for the formation of an inorganic-dominated, robust interphase layer on LCO and graphite electrodes, resulting in outstanding cycling stability in half and full cells.
While the LiPF6 in current use decomposes in the presence of moisture, releasing highly toxic and corrosive hydrogen fluoride gas (HF), LiDFOB is water- and air-stable. Instead of strict dry room conditions, LIBs with LiDFOB can be made under ambient conditions—an additional cost-saving feature. According to the researchers, recycling could also be significantly less problematic, leading to improved sustainability.
- An Ultralow‐concentration and Moisture‐resistant Electrolyte of Lithium Difluoro(oxalato)borate in Carbonate Solvents for Stable Cycling in Practical Lithium‐ion Batteries,
Zhishan Liu, Wentao Hou, Haoran Tian, Qian Qiu, Irfan Ullah, Shen Qiu, Wei Sun, Qian Yu, Jinliang Yuan, Lan Xia, Xianyong Wu,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202400110