Antonio M. Echavarren and colleagues, Institute of Chemical Research of Catalonia (ICIQ, CERCA), Tarragona, Spain, and Universitat Rovira i Virgili, Tarragona, Spain, have shown why some gold(I)-catalyzed alkoxycyclizations produce products with very different enantiomeric ratios, even when formed in the same reaction.
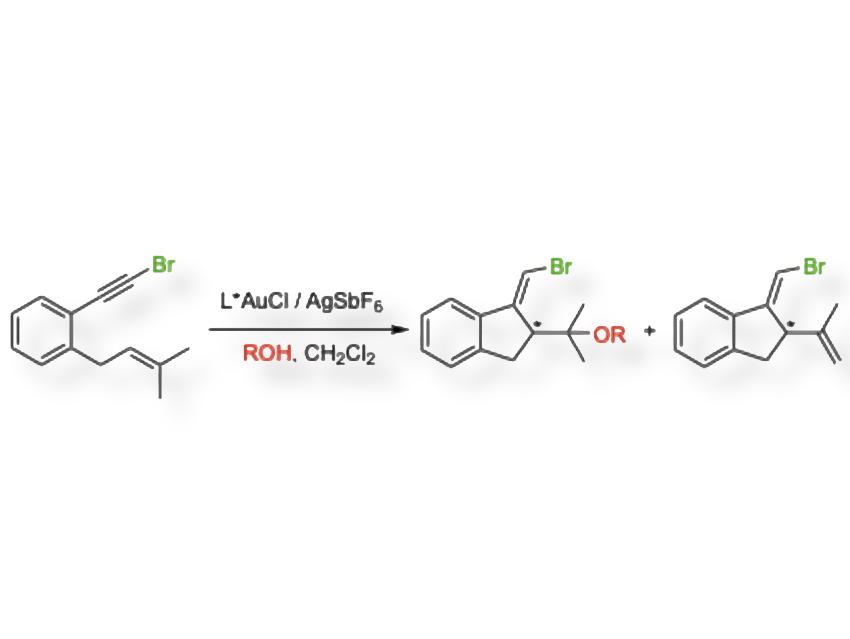
The team has developed the first enantioselective gold(I)-catalyzed cyclizations of bromo-1,5- and bromo-1,6-enynes (pictured), including spiro compounds, using specially designed chiral phosphine ligands. They combined experiments with DFT calculations to understand how the reaction proceeds and why selectivity changes under different conditions.
The researchers observed that a key step of the reaction can be reversible. When this happens, part of the reaction mixture can racemize, explaining why an alkoxycyclization product is enantioenriched while the cycloisomerization product is almost racemic. The selectivity also depends on the amount of methanol present: low quantities make the hydride shift competitive, while higher amounts speed up the alkoxycyclization and lead to enantioenriched products.
In more detail: The gold(I) catalyst binds to the alkyne part of the enyne, making it more reactive and ready to cyclize. The alkene attacks the activated alkyne, creating a new carbon–carbon bond and forming a cyclic, positively charged intermediate. The chiral ligand around gold controls from which side the ring forms, so one mirror image (enantiomer) is favored at this stage. Then two pathways compete after cyclization: a) If an alcohol is present, it quickly reacts with the intermediate, “locking in” the chirality and giving an enantioenriched product. b) If no alcohol is present, the intermediate can rearrange via a 1,2-hydrogen shift, which scrambles the chirality.
The results help explain the mechanisms behind one of the most representative transformations in gold(I) catalysis and shed new light on the complex mechanisms of gold(I)-catalyzed alkoxycyclizations, the researchers say. The study also highlights the importance of highly polarized haloalkynes, versatile building blocks widely used in synthetic chemistry whose dual reactivity allows further functionalization and efficient construction of complex structures.
- Enantioselective Cyclization of Bromoenynes: Mechanistic Understanding of Gold(I)-Catalyzed Alkoxycyclizations
A. Cataffo, E. García-Padilla, I. Escofet, N. Fincias, A. Arnanz, G. Zuccarello, G. Tian, L. Cai, F. Khorasani, F. Maseras, A. M. Echavarren
Chem. Sci. 2026.
https://doi.org/10.1039/D5SC09023G