Due to its toxicity, the chemistry of beryllium is poorly understood. Since the lightest elements form the basis for fundamental models of chemical bonding, there is a need for better insight into the properties of beryllium, and here the chemistry of the homo-elemental Be–Be bond is of particular interest.
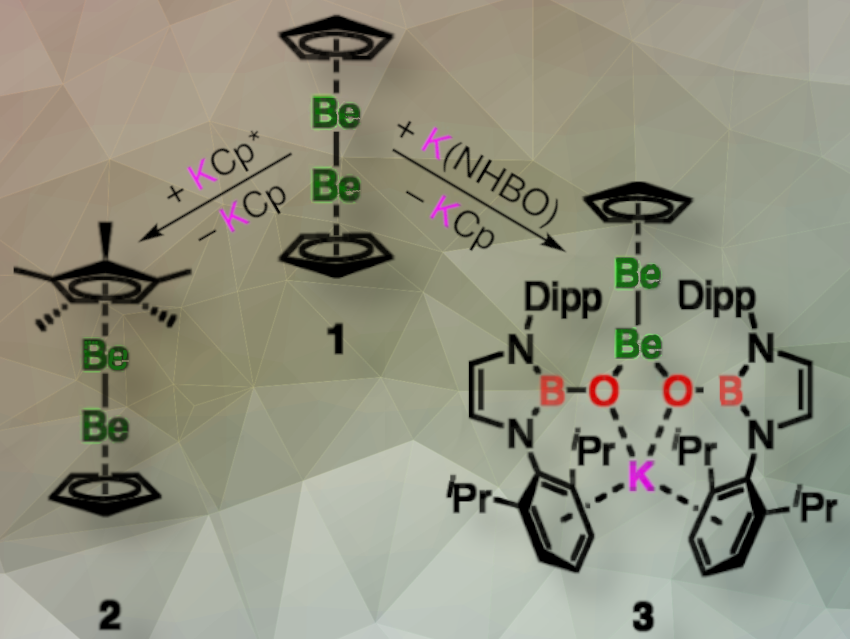
Josef T. Boronski, Simon Aldridge and colleagues, University of Oxford, UK, have investigated the ligand metathesis chemistry of diberyllocene (CpBeBeCp, 1), a stable complex with a Be–Be bond. They have synthesized two new complexes with Be–Be bonds: Cp*BeBeCp (2) and [K{(HCDippN)2BO}2]BeBeCp (3; Dipp = 2,6-diisopropylphenyl). Ligand metathesis at a [Mg–Mg]2+ unit has not been reported before. The ability of complex 1 to undergo ligand metathesis while retaining the Be–Be bond, supports the chemical similarities between [Zn–Zn]2+ and [Be–Be]2+ units.
The complexes 2 and 3 are both unsymmetrical and thus have Be–Be bonds that are polarized, although to very different degrees. Quantum chemical calculations suggest that 3 can be formulated as a mixed-valence Be0/BeII complex, which is supported by experimental evidence such as single crystal X-ray diffraction (SCXRD) measurements and reactivity studies.
In particular, complex 3 can transfer a beryllyl anion ([BeCp]–) to an electrophilic organic substrate. The reaction of complex 3 with [CPh3][B(C6F5)4] produces previously unreported complexes Be(NHBO)2 and CpBe(CPh3), as well as K[B(C6F5)4], through the transfer of the nucleophilic [CpBe]− anion. This reactivity can be compared to that of sp2-sp3 diboranes. Despite the respective metallic and non-metallic nature of these elements, the reactivities of the homo-elemental bonds of beryllium and boron have parallels, and according to the researchers, this work shows that the bonding trends over period 2 are more continuous than may have been previously appreciated.
- A nucleophilic beryllyl complex via metathesis at [Be–Be]2+,
Josef T. Boronski, Agamemnon E. Crumpton, Aisling F. Roper, Simon Aldridge,
Nature Chem. 2024.
https://doi.org/10.1038/s41557-024-01534-9