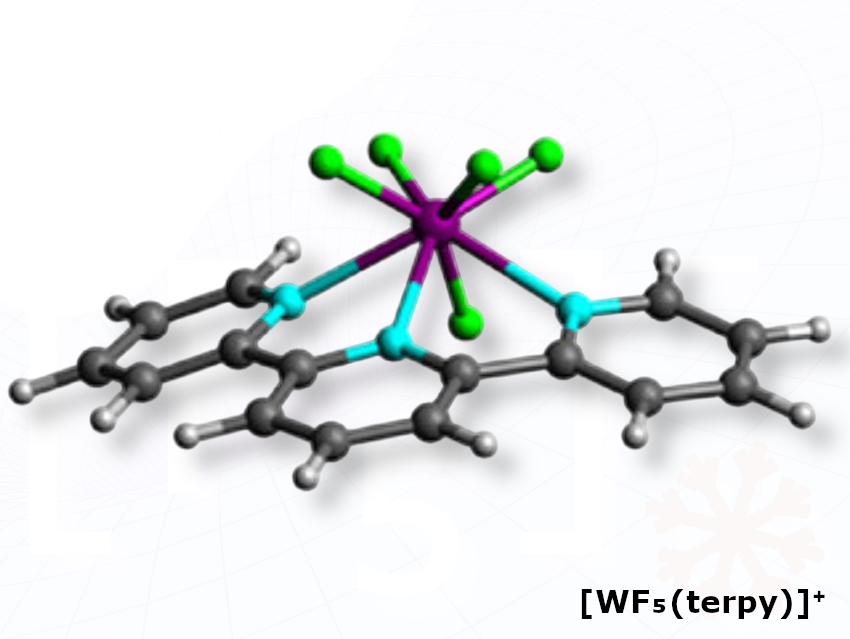
Michael Gerken, University of Lethbridge, Canada, and colleagues have reacted molybdenum and tungsten hexafluorides with the tridentate ligand terpyridine (terpy), causing the molecules to split unevenly and form paired ions instead of simple neutral adducts.The team reacted MoF₆ in CH₂Cl₂ and WF₆ in liquid SO₂ with terpyridine, which caused each hexafluoride to autoionize and form the paired salts [MoF₅(terpy)][MoF₇] and [WF₅(terpy)][WF₇]. The researchers also report that the tungsten salt could be further treated with SbF₅·SO₂ to replace the [WF₇]⁻ anion with [SbF₆]⁻, whereas the analogous molybdenum reaction led instead to reduction of Mo(VI).
The researchers report that the metal–fluoride–terpyridine cations adopt an uncommon “bicapped trigonal prism” shape. The molybdenum product [MoF₅(terpy)]⁺ represents the first known cationic Mo(VI)–fluoride complex.
The team notes that this unusual autoionization pathway and rare geometry expand the known chemistry of high-valent fluorides, offering new ways to stabilize highly reactive metal centers.
- Synthesis and Characterization of [MF₅(terpy)]⁺ (M = Mo, W) Salts: Autoionization of MF6 Induced by a Tridentate Ligand
Miriam D. van Hoeve, Taylor P. K. Adamitz, Felix O’Donnell, Stacey D. Wetmore, Michael Gerken
Chem. Eur. J. 2025
https://doi.org/10.1002/chem.202502997