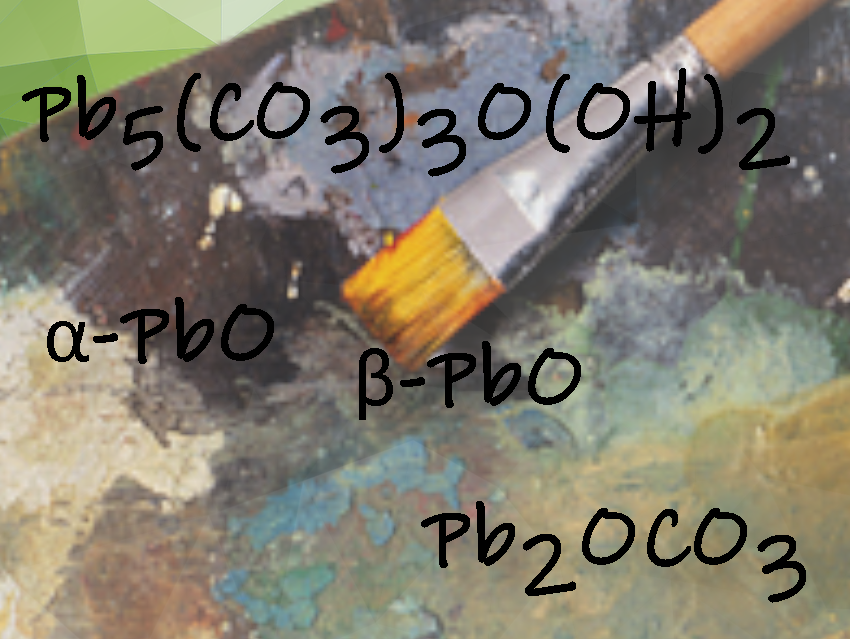In the early 16th century, including Leonardo da Vinci’s “Mona Lisa”, many paintings were done on dark wooden panels. To enhance color vibrancy, artists commonly applied a thick white ground layer, typically made of a mixture known as Gesso, which included glue, gypsum (CaSO4) or chalk (CaCO3), and white pigment. However, Leonardo used unconventional materials, like lead-based pigments (PbCO3, Pb3(CO3)2(OH)2, Pb3O4), enriching his oil with lead oxide (PbO), as evidenced by non-invasive techniques.
Victor Gonzalez, Université Paris-Saclay, ENS Paris-Saclay, Gif-sur-Yvette, France, PSL Research University, Paris, and Centre de Recherche et de Restauration des Musées de France, C2RMF, Palais du Louvre, Paris, France, and colleagues have analyzed microsamples from the ground layer of Leonardo da Vinci’s Mona Lisa, painted ca. 1503–1519 and housed in the Louvre in Paris, and 17 microsamples from his “Last Supper“, painted ca. 1494–1498 and housed in the Santa Maria delle Grazie monastery in Milan, Italy. They used synchrotron radiation high-angular resolution X-ray powder diffraction (SR-HR-XRPD), synchrotron radiation micro X-ray powder diffraction (SR-μ-XRPD), and micro Fourier transform infrared spectroscopy (μ-FTIR) to reveal the exact compositions. They compared their findings with Leonardo da Vinci’s historical records of his work.
The ground layers of the artworks were found to contain a highly saponified oil with a high lead content and a lead white pigment enriched with cerussite (PbCO3) and hydrocerussite (Pb3(CO3)2(OH)2), as well as some calcite (CaCO3). The researchers also discovered a small amount of a rare plumbon-acrite (Pb5(CO3)O(OH)2). Plumbonacrite is stable only under alkaline conditions, suggesting that this material was formed by a reaction between the oil and added lead(II) oxide (PbO).
In the samples from the “Last Supper”, the researchers also found PbO grains of orange litharge (α-PbO) and yellow massicot (β-PbO) in the primer and the overlying paint layer, formed at different temperatures. They also identified the mineral shannonite (Pb2OCO3) in the mural, potentially serving as an intermediate form in the formation of plumbonacrite from PbO.
Plumbonacrite had previously been found in paintings by the 16th century Dutch Baroque artist Rembrandt van Rijn. Painters of his era were known to add lead compounds such as plumbonacrite to their pigments to help them dry or harden. However, when da Vinci began painting the “Mona Lisa” around 1503, such priming techniques were thought to be uncommon in the Italian Renaissance. The lead-containing mineral shannonite was found for the first time in a historical painting, and its impact on colors and whether it is a precursor of plumbonacrite remain unclear.
These findings suggest that lead oxides were a consistent feature in the palettes of the old masters, including Leonardo da Vinci. Further research will delve into how this primer mixture influenced the colors in his works and whether similar compositions exist in other works of da Vinci and his contemporaries.
- X-ray and Infrared Microanalyses of Mona Lisa’s Ground Layer and Significance Regarding Leonardo da Vinci’s Palette,
Victor Gonzalez, Gilles Wallez, Elisabeth Ravaud, Myriam Eveno, Ida Fazlic, Tiphaine Fabris, Austin Nevin, Thomas Calligaro, Michel Menu, Vincent Delieuvin, Marine Cotte,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c07000
Also of Interest
 |
Video: How Do Oil Paints Dry?, |

A compilation of articles on chemistry in artworks, on art inspired by chemistry, about the work of scientific teams in museums
The reason behind Mona Lisa’s enigmatic smile has been exposed as X-ray fluorescence spectroscopy unveils Da Vinci’s sfumato technique

Identification of chemical reaction responsible for the degradation of two paintings by Vincent van Gogh could help in their preservation