Gabriel Merino, Unidad Mérida, Maxico, discusses a recent study published by him and colleagues in Chemistry – A European Journal on whether planar hypercoordinate atoms beyond carbon could really be stabilized in heavier elements.
What did you do?
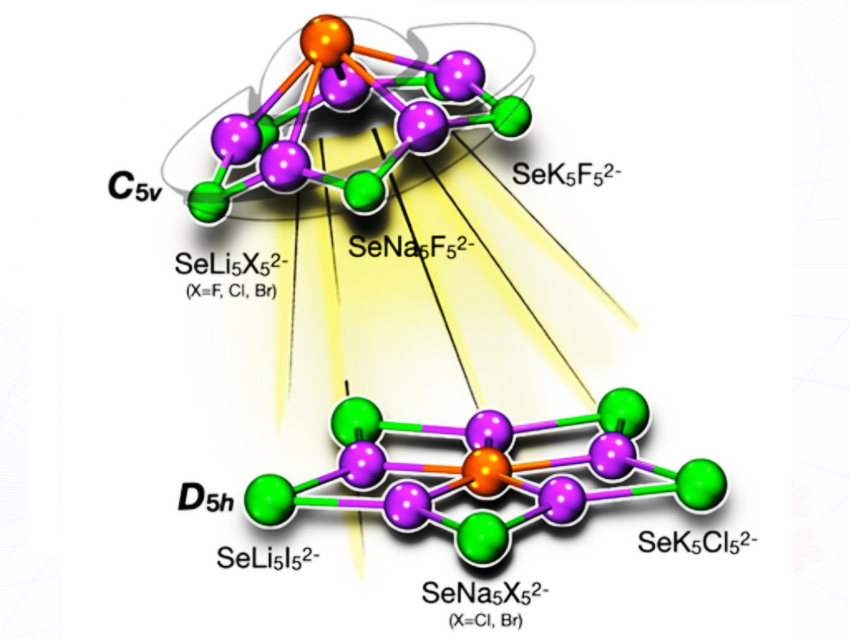
We used quantum chemical calculations to explore whether selenium atoms can adopt unusual pentacoordinate geometries, specifically planar and pyramidal arrangements, in dianionic systems of the type SeM₅X₅²⁻ (M = alkali metals, X = halogens).
What interests you about this?
Chemists are fascinated by “forbidden” or counterintuitive structural patterns. Just as planar tetracoordinate carbon challenged classical rules, we wanted to test whether selenium can also break conventional coordination limits, expanding our understanding of chemical structure and bonding in heavier main-group elements.
What is new and cool about your research?
We showed that selenium can be stabilized in a perfectly planar pentacoordinate environment, something never reported before. This pushes the boundaries of what geometries atoms can adopt and shows how electrostatics and geometric confinement can override traditional bonding expectations.
What else did you find?
Our main finding is that selenium, a relatively heavy main group element, can adopt both planar and pyramidal pentacoordinate geometries. We also quantified how electrostatic interactions, cavity size, and ligand arrangement interact to stabilize these unusual structures. In other words, we identified the geometric and electronic conditions that allow selenium to remain planar rather than collapse into its more conventional pyramidal shape.
The bonding and stability are not driven by aromaticity but by ligand-selenium electrostatic interactions and spatial constraints.
What is your longer-term vision?
While these are theoretical species, they could inspire:
- The design of novel materials with unusual electronic or magnetic properties.
- New conceptual models for understanding bonding in heavier main‑group elements.
- Guidelines for synthetic chemists aiming to stabilize exotic structures in the lab.
What part of your work was the most challenging?
The most challenging aspect was forcing a selenium atom to remain in a planar five-coordinate arrangement, a geometry it doesn’t naturally adopt. Selenium normally prefers three-dimensional structures, so even small electronic imbalances tend to push it out of the plane. From a computational perspective, this meant exploring various configurations and then verifying the stability of each candidate using several density functional approximations and basis sets to ensure that the planarity was not simply a modeling artifact.
What is the broader significance of theoretically predicting new molecules in chemistry?
This work shows that chemical imagination plus computation can uncover new bonding possibilities. Even if these molecules have not yet been synthesized, they expand the conceptual toolkit of chemistry and challenge us to rethink the limits of atomic coordination.
Thank you very much for sharing these insights.
The paper they talked about:
- Planar and Pyramidal Pentacoordinate Selenium Atoms,
Luz Diego, Alejandro Vásquez-Espinal, Rafael Islas, Gabriel Merino,
Chem. Eur. J. 2025.
https://doi.org/10.1002/chem.202502525
Gabriel Merino is a professor in the Departamento de Física Aplicada at the Centro de Investigación y de Estudios Avanzados (CINVESTAV), Unidad Mérida, Mexico.
Also of Interest