Crown ethers are macrocyclic chelating ligands. They can be used, e.g., to improve the solubility of compounds or to capture specific ions. Crown ethers are used often, but comparatively little is known about the binding of actinides in these ligands—in particular, about the properties of crown-ether-chelated uranyl complexes in solution.
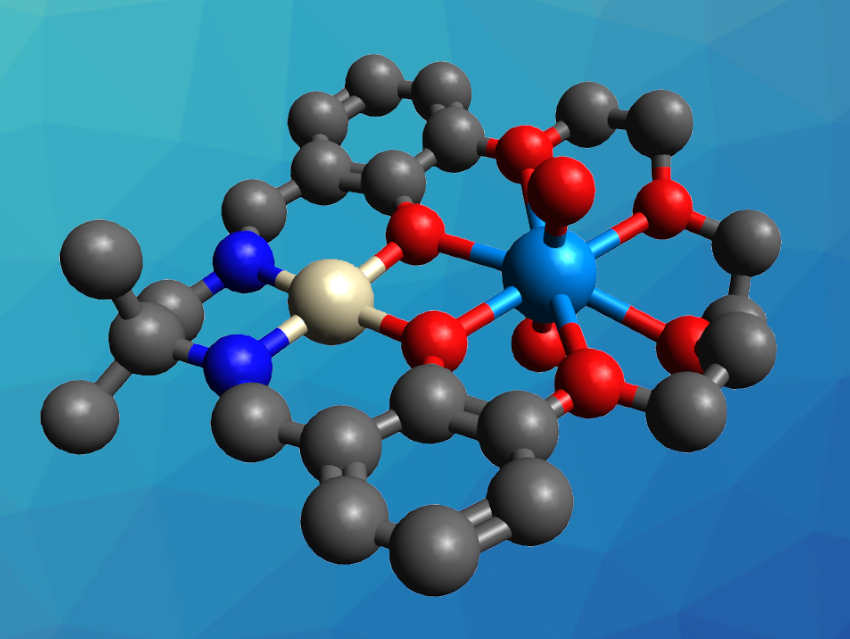
Vassiliki-Alexandra Glezakoun, Oak Ridge National Laboratory, TN, USA, James D. Blakemore, University of Kansas, Lawrence, USA, and colleagues have synthesized heterobimetallic uranyl crown ether complexes (example pictured, U in light blue, Pt in light grey). The team used a macrocyclic ligand with a “pre-installed” Pt(II) center to form a binding site that resembles that of 18-crown-6. They started from a barium complex that serves as a precursor, which was converted to the desired platinum complex using Ba(OH)2 as a base and Pt(DMSO)2Cl2 (DMSO = dimethyl sulfoxide) as the Pt source.
The Pt-containing ligand was then used to capture UO22+ via a reaction with anhydrous uranyl triflate (UO2(OTf)2) in acetonitrile. The resulting heterobimetallic complex was characterized using single-crystal X-ray diffraction, which confirmed that the uranyl ion is bound in the crown-ether-like site of the ligand. The researchers studied the redox properties of the complex using electrochemical experiments and observed the formation of a U(V) complex. Both the U(VI) and U(V) forms of the uranyl ion were stably bound by the crown-ether-like ligand.
- Synthesis, Isolation, and Study of Heterobimetallic Uranyl Crown Ether Complexes,
Riddhi R. Golwankar, Alexander C. Ervin, Małgorzata Z. Makoś, Emily R. Mikeska, Vassiliki-Alexandra Glezakou, James D. Blakemore,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c12075