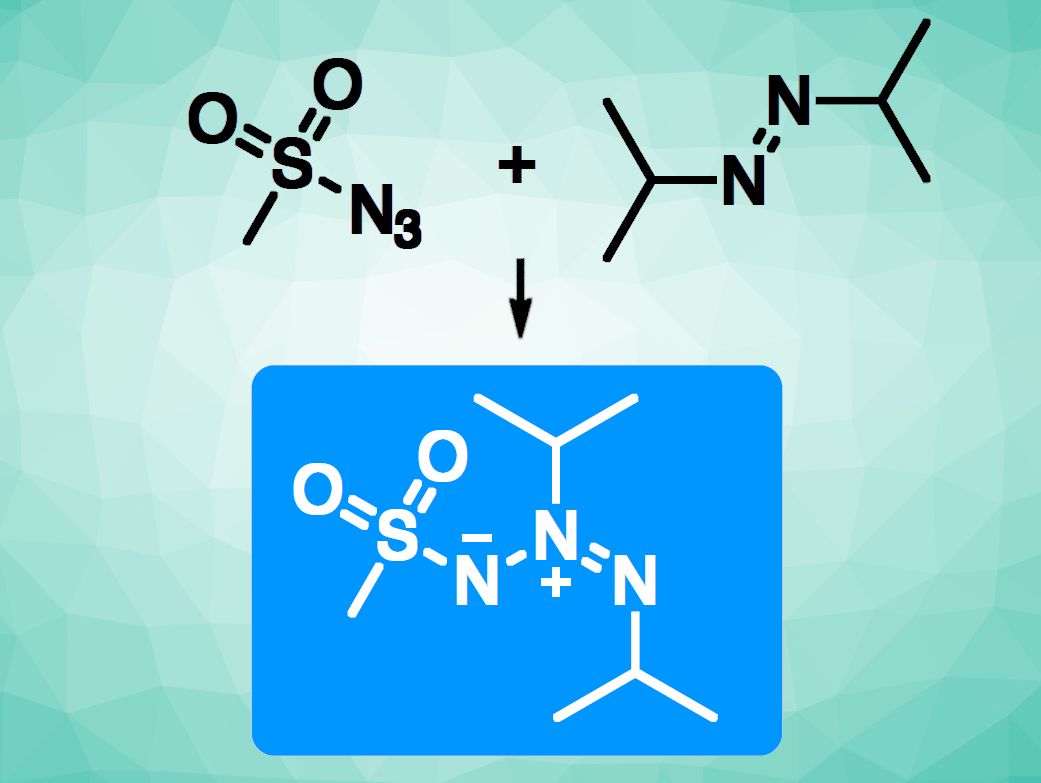
The use of visible light—the ultimate renewable source of energy—to enable organic reactions is a useful development. Azimines (or azo imides, example pictured in the blue box) are all-nitrogen 1,3-dipolar compounds, which can be useful, e.g., in cycloaddition reactions, as dyes, or in energetic materials. One path to azimines involves the reaction of a nitrene with an azo compound. However, this approach suffers from poor atom economy and uses stoichiometric conditions. A catalytic, light-driven approach could be a useful alternative.
Lionel Joucla, Emmanuel Lacôte, Université Claude Bernard Lyon 1, CNRS, CNES, ArianeGroup, LHCEP, Villeurbanne, France, and colleagues have developed a mild photocatalytic method to access azimines from sulfonyl azides and dialkyldiazenes (example pictured). The team reacted a variety of sulfonyl azides (RSO2N3) with different electron-rich azo reagents (R’N=NR’) in the presence of the iridium photocatalyst [Ir(dmppy)2dtbbpy]PF6 (dmppy = dimethylphenylpyridin, dtbbpy = di-tert-butyldipyridine) under blue LED light at room temperature, using CH2Cl2 as the solvent. The desired products were obtained in moderate to good yields.
The team proposes that the reaction might proceed via the sensitization of the azo reagent, which undergoes a tandem dipolar cycloaddition/retro cycloaddition with the azide. The method shows that photocatalysis can be harnessed to construct N–N bonds, which can be a challenging task. Interestingly, the reaction could be implemented in a continuous-flow process. This is an important feature from an industrial and safety standpoint because poly-nitrogenated structures can be hazardous and impact/friction sensitive.
- Mild Photocatalytic Synthesis of Azimines,
Emma Gamby, François Liger, Lionel Joucla, Emmanuel Lacote,
Eur. J. Org. Chem. 2022.
https://doi.org/10.1002/ejoc.202201071