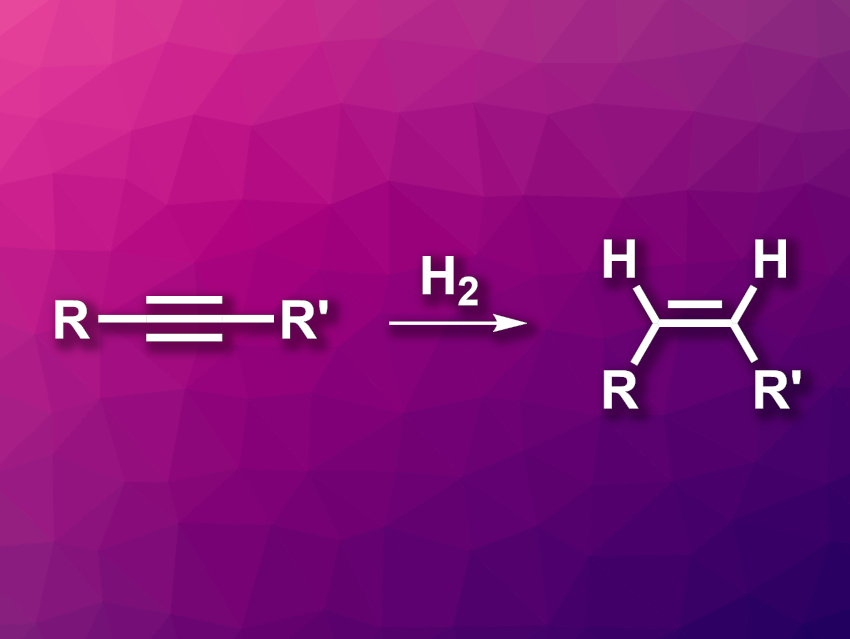
The activation of small molecules such as H2 is an interesting research target. Transition-metal complexes, for example, can be used for this type of activation, and there have also been developments in the use of main-group compounds. The activation of dihydrogen with zinc-based catalysts has received less attention, and the substrate scope and selectivity of hydrogenations using Zn can be limited.
Ian J. Casely, Johnson Matthey Technology Centre, Reading, UK, Damian Grainger, Johnson Matthey, Cambridge, UK, Mark R. Crimmin, Imperial College London, UK, and colleagues have developed a method for the activation of dihydrogen with a zinc anilide complex (pictured below), which can be used for the selective semi-hydrogenation of alkynes (pictured above). The team synthesized the zinc anilide complex via a stepwise reaction of ZnCl2 with the lithiated form of the ligand and LiNHPh.
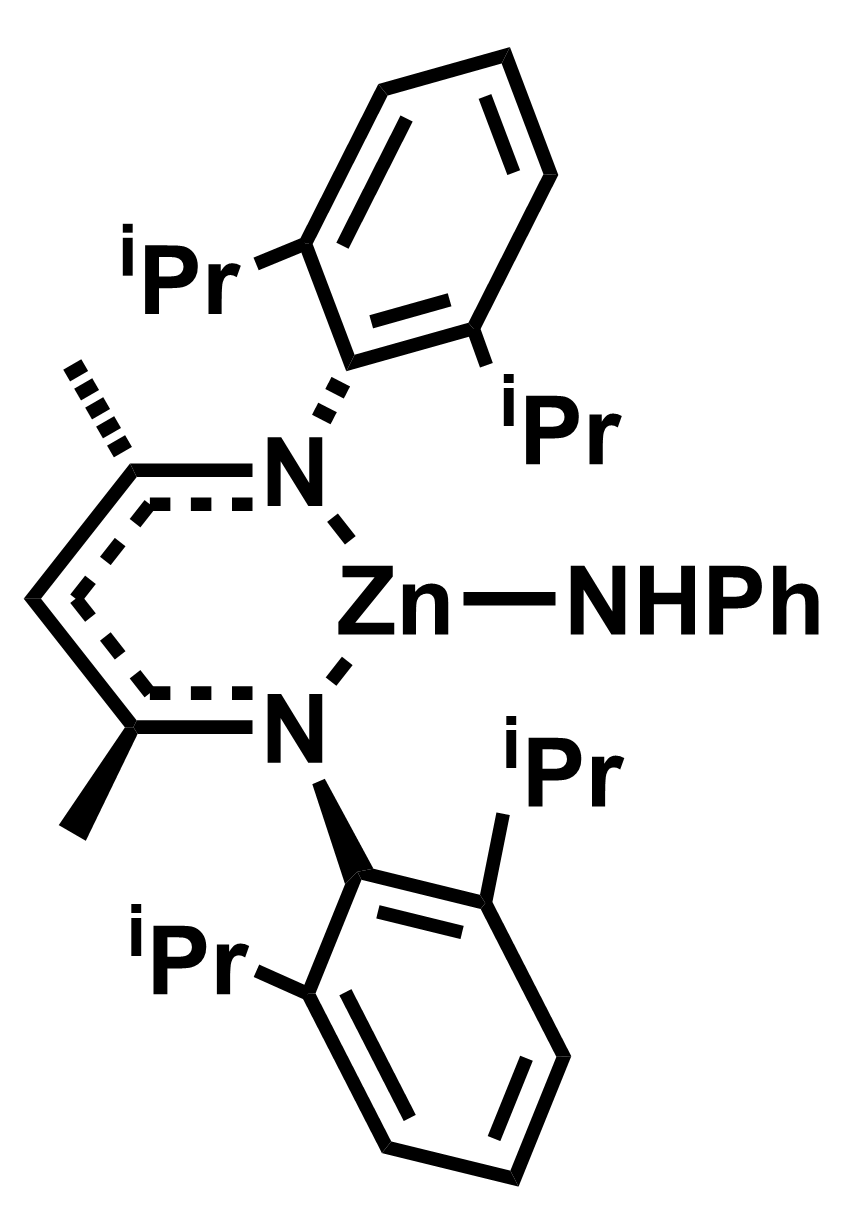
The team found that the complex can reversibly activate H2 via an addition across a Zn–N bond, giving a zinc hydride. This zinc hydride can be used for the hydrozincation of unsaturated substrates. For alkyne substrates, this hydrozincation selectively gives the syn-isomer. This can be used for the stereoselective semi-hydrogenation of different alkynes to form Z-alkenes. The semi-hydrogenation reactions were performed under 23 bar H2 at 145 °C.
- Catalytic, Z-Selective, Semi-Hydrogenation of Alkynes with a Zinc–Anilide Complex,
Greg J. Baker, Andrew J. P. White, Ian J. Casely, Damian Grainger, Mark R. Crimmin,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c02301