Title: Planar Tetracoordinate Fluorine in Binary Clusters
Authors: Kangkan Sarmah, Li-Xia Bai, Jin-Chang Guo, Ankur Kanti Guha
Published: 2 June 2025 in ChemPhysChem
🔬 What They Did
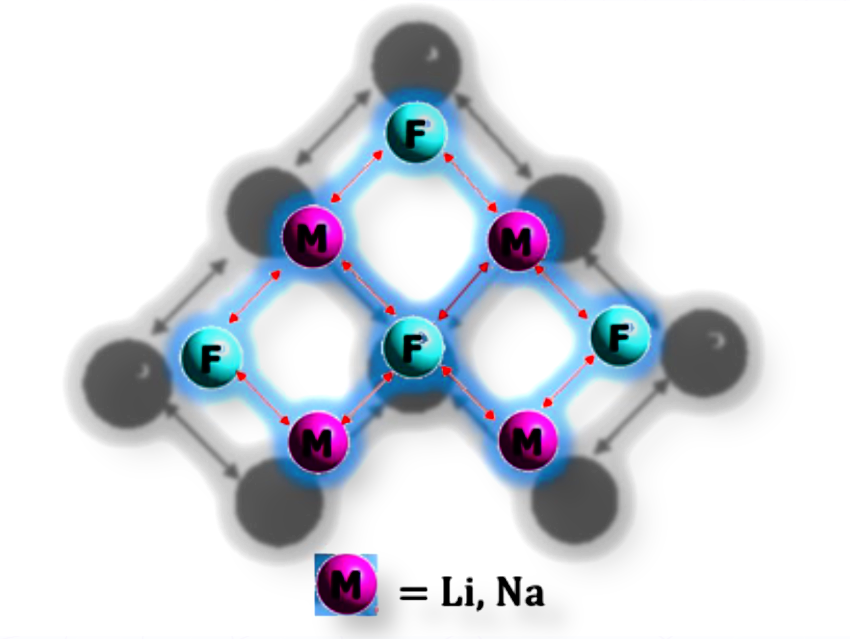
Using density functional theory (DFT) and global minimum structure searches, the authors predicted the first examples of planar tetracoordinate fluorine (ptF) in Li₄F₄⁻ and Na₄F₄⁻ clusters with C₂ᵥ symmetry.
Despite the long list of planar hypercoordinate maingroup elements, fluorine has shown resistance due to its high
electronegativity.
🔍 What They Found?
The planar geometry is stabilized not by covalent delocalization, as in classic planar tetracoordinate carbon species, but by strong electrostatic attraction and multicenter ionic bonding between F⁻ and surrounding alkali metals. The clusters showed dynamic and thermodynamic stability in Born–Oppenheimer molecular dynamics and fragmentation analyses.
🌍 Why It Matters
This work establishes a new bonding paradigm for highly electronegative elements like fluorine and offers a blueprint for designing unconventional molecular architectures.
🧩 Cool Detail
The 18-valence-electron rule often guides planar hypercoordinate design, but exceptions exist. For instance, 17-electron CAl₄⁻ shows planar geometry, and CBe₅H₄⁺ (17e⁻) is planar, while its 18e⁻ neutral form adopts a tetrahedral shape.