Secondary amines and imines are ubiquitous functionalities throughout organic synthesis and pharmaceutical chemistry. Amine dehydrogenation is an underdeveloped method to access such groups. N–H bond activation is a key step involved in amine dehydrogenation and is typically induced by a Lewis base. Thus, suitable metal catalysts for amine hydrogenation would benefit from a Lewis basic site as well as a labile ligand, but should also avoid an undesired Lewis acid-base pair.
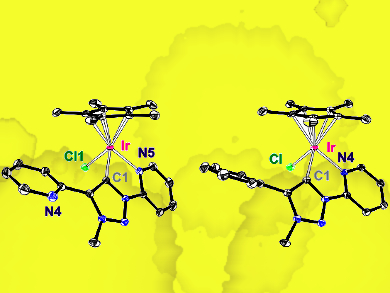
Martin Albrecht, University of Bern, Switzerland, and colleagues have designed and synthesized a triazolylidene ligand containing a pendant pyridine unit suitable for both dehydrogenation and N–H bond activation. Upon coordination to iridium, a chelate-stabilized complex is formed. It facilitates dehydrogenation and the pyridine unit undergoes hydrogen bonding allowing for N–H bond cleavage. The strain caused by the five-membered ring of the ligand allows for only one of two possible pyridine units to bind to the metal.
Iridium complexes were synthesized from the ligand and iridium precursors (pictured) and characterized by both NMR and X-ray crystallography, confirming the structures. The complexes were then used successfully as catalysts for the oxidative coupling of benzylic amines under base- and oxidant-free conditions. They also catalyzed the reverse reaction, the hydrogenation of imines, making these interesting dual catalysts for related applications. Stoichiometric experiments showed the metal-ligand cooperativity of these complexes, with reactivity depending on the availability of the pendant pyridyl unit.
- Triazolylidene-Iridium Complexes with a Pendant Pyridyl Group for Cooperative Metal-Ligand Induced Catalytic Dehydrogenation of Amines,
Marta Valencia, Ana Pereira, Helge Müller-Bunz, Tomás R. Belderraín, Pedro J. Pérez, Martin Albrecht,
Chem. Europ. J. 2017, 23, 8901–8911.
DOI: 10.1002/chem.201700676