Impressive and precious textiles, embroideries, and traditional costumes can be found in museum and private collections. The identification of their chemical constitution, as well as the conditions under which that might be influenced, play an important role in their effective conservation [1].
Generally speaking, textiles are influenced by various factors such as light, atmospheric pollution, insects, and certain microorganisms, which can individually or synergistically cause important loss of their integrity and finally their destruction [2–6]. The rate of decomposition depends to a large extent on the textiles’ environment. However, the nature of the fibers should not be ignored. Over the last 50 years, a large number of synthetic polymers has been used in the conservation of historical textiles where they act as consolidants or adhesives.
1 Introduction
Fibers are flexible polymeric materials with lengths that are at least 100 times larger than their widths. From this definition, it becomes clear that to have fibers with satisfactory mechanical strength, it is necessary to have a macromolecular structure. Depending on their origin, fibers are distinguished as being either natural (wool, silk, cotton, etc.) or man-made (rayon, viscose, polyamide, polyester, polyacrylonitrile, etc.) (see Tab. 1 and Fig. 1).
Table 1. Classification of common fibers.
|
|
||||
|
Natural Fibers |
|
Man-Made Fibers |
||
|
|
||||
|
Animal |
Vegetable |
|
Modified natural polymers |
Synthetic organic polymers |
|
|
||||
|
Wool |
Cotton |
|
Rayon viscose Cellulose acetate Alginic fibers |
Polyamides (nylon) Polyesters Polyacrylonitriles Polyurethanes Polyethene Polypropene Polytetrafluoroethene |
|
|
||||
Animal fibers (see Fig. 1) are based on the keratin protein, whereas silk is based on the protein fibroin. Wool is characterized as the fur of sheep, whereas the fur of other animals is characterized with other names. Usually, the fibers take the name of the animal from which they come. Vegetable fibers are cellulose and come from different parts, such as bast, leaves, seeds, of specific plants.
Of the modified natural polymers, the most common are modified cellulose fibers, such as rayon or viscose and cellulose acetate. From the beginning of the 20th century, rayon managed to be a substitute for vegetable fibers with great success, but after 1950 the appearance of man-made fibers caused a significant reduction in its production.

Figure 1. Microscopic images of (a) cotton (image by Featheredtar, CC-BY 3.0), (b) wool (image by Gerry Danilatos, CC-BY-SA 3.0), and (c) nylon.
2 Conservation of Textiles
Textiles are an expression of the culture and personality of people. When they become works of art, it is desirable to keep them unchanged for many years. This is something that is not always feasible. Textiles from animals and vegetables can be attacked by bacteria and mold. Others can deteriorate or be damaged after long-term exposure to air.
The main factors that cause the degradation of textiles are light (visible and ultraviolet), temperature, humidity, microorganisms, and the exposure to acids and bases. Some of these factors act synergistically to accelerate the decomposition. An example is the combination of high temperature and high relative humidity, which can cause the development of microorganisms and mold and change the color of a textile from white to gray. The presence of a low quantity of metals like copper can accelerate the degradation of the fiber under the presence of ultraviolet radiation and humidity. Additionally, a huge amount of damage can be caused by large fluctuations in temperature and relative humidity. This happens because hydrophilic fibers very easily absorb or lose moisture and thereby change their dimensions.
As a result of the textile degradation, there is a breaking of the macromolecular chains of the fiber and a gradual loss of the endogenous humidity. The fibers become more fragile, less elastic, and weaker. For these reasons, most museums try to maintain a temperature of about 18 °C and a relative humidity (RH) of 55 %.
Light can have dramatic consequences on textiles and their pigments. Visible and ultraviolet light have the necessary energy to activate chemical reactions that can lead to textile degradation in the presence of moisture and oxygen. Irradiation with ultraviolet light accelerates the fading of the fibers and at the same time, the fibers become particularly fragile. Additionally, dust and atmospheric pollutants contain a high level of thin sand grains. The sharp surfaces of the dust can damage textile fibers, as the fibers contract and expand upon changes of relative humidity in the environment. Finally, sulfur dioxide in parallel with discoloration can make the fibers more fragile.
Conservators try to understand the changes occurring in textiles so that they can try to stabilize them or even stick them on a stable substrate to slow the decomposition processes that take place.
3 Consolidation of Textiles
The purpose of consolidation is to hold together the degradable and strained fibers and to give strength to the textiles. During consolidation, a suitable substance (consolidant) in solution is inserted between the fiber and after the removal of the solvent, consolidation is achieved. With this process, the textile becomes stronger and more resistant and if it is handled with care (in a museum collection, for example) it can remain unchangeable for many years.
Many consolidants have been used over time, like cellulose nitrate, natural resins, rubber solutions, or latex in chloroform together with cellulose nitrate and linseed oil. Cellulose fibers (Modocoll) have been applied in the consolidation of cellulosic fibers, whereas solutions in ethanol of water-soluble poly(vinyl alcohol) (PVAl) and polyvinyl butyral (PVB) have been used since 1960. Low-molecular-weight polyethylene glycol (PEG), which has been used in the consolidation of old textiles, is known to darken the textiles; it is very hygroscopic and incorporates dust and other types of pollutant.
In [7], the case of a silk textile is mentioned, saturated with PVAl or PVB. A textile, under the same conditions of aging, showed a bigger reduction of its mechanical properties in relation to a textile that had not been subjected to the respective conservation treatment. The flexibility of the modified fibers is usually reduced by the aging of the consolidant.
During the aging of PVAl, PVB, or nylon, cross-links are formed that transform the flexible linear polymer into a polymeric rigid net with reduced flexibility and solubility (see Fig. 2).
Figure 2. Cross-linking chemical reaction of PVAl.
An ideal consolidant should have the following characteristics:
- It should give strength and in some cases flexibility to the decayed and brittle fibers, so that they can endure fission and breakage.
- The process of conservation should be reversible and compatible with the work of art, and the consolidation should be chemically stable over long-term time periods and should not cause any damage to the textile.
- The consolidant must be resistant to environmental factors such as ultraviolet radiation, insects, and so forth.
- It should not change the color, texture, or appearance of the fiber.
- It should have low viscosity, good penetration, and cause the least shrinkage upon the loss of the solvent.
- It should not cause swelling of the fibers.
4 Adhesion of the Textile
During the conservation of a textile, a liquid adhesive can be applied upon a polyester textile substrate, for example, and be left to dry. Next, the work of art is layered upon the substrate and the adhesion of the two surfaces is accomplished. For this purpose, the adhesive should soften so that it can provide very good contact with the back surface of the textile. Heating helps the adhesion process.
Most of the adhesives that have been applied to historical textiles were solutions, dispersions, or emulsions of long-chain synthetic polymers. Examples for the use of mixtures of natural and synthetic adhesives can be found in [8].
5 Properties of Consolidants and Adhesives
The majority of the consolidants and adhesives that have been used in the conservation of textiles are polymers. Beyond the conditions of heating, the state of a polymer (liquid or solid) is affected to a large extent by the degree of polymerization, and, furthermore, by the mechanical properties. In Table 2, some of the polymers used in the conservation of textiles are presented. In Table 3 the most often used synthetic polymers with their trade names are shown.
The majority of the synthetic polymers used as adhesives are polymerized from monomers that have carbon–carbon double bonds (in unsaturated compounds). These compounds often have vinyl groups like ethene, propene, acetic vinyl ester, and acrylic acid.
Table 2. Some of the polymers used in the conservation of textiles.
Table 3. Trade names of polymers used in the conservation of textiles.
|
|
|
|
Polymer |
Monomer |
|
|
|
|
Lascaux 360HV (Lascaux) |
>50 % butyl methacrylate, other acrylates |
|
Lascaux 498HV (Lascaux) |
56 % butyl methacrylate, other acrylates |
|
Mowilith DMC2 (Hoechst) |
65 % vinyl acetate, 35 % di-n-butyl maleate |
|
Mowilith DMC5 (Hoechst) |
65 % vinyl acetate, 35 % butyl acrylate |
|
Paraloid B72 (Rohm and Haas) |
70 % ethyl methacrylate, 30 % methyl acrylate |
|
Paraloid F10 (Rohm and Haas) |
n-butyl methacrylate |
|
Plextol B500 (Rohm GmbH) |
≈60 % ethyl acrylate, ≈40 % methyl methacrylate, x % ethyl methacrylate |
|
Primal AC-33 (Rohm and Haas) |
≈60 % ethyl acrylate, ≈40 % methyl methacrylate, x % ethyl methacrylate |
|
Texicryl 13-002 (Vinyl Products |
≈35 % methyl methacrylate, ≈65 % ethyl acrylate, x % ethyl methacrylate |
|
Vinamul 3252 (Vinyl Products) |
50 % ethylene, 50 % vinyl acetate |
|
Vinamul 325 (Vinyl Products) |
45 % ethylene, 55 % vinyl acetate |
|
Vinnapas EP-1 (Wacker) |
20 % ethylene, 80 % vinyl acetate |
|
|
|
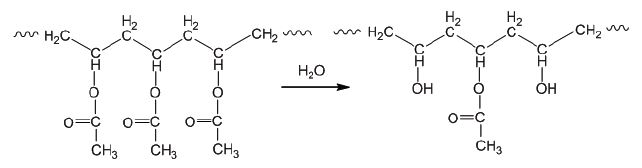
Poly(vinyl alcohol), which is known on the market as Mowiol or Rhodoviol, cannot be produced from the polymerization of monomers because vinyl alcohol is a very unstable intermediate product that is found in some chemical reactions but not in the form of a monomer. Therefore, it is produced from poly(acetic vinyl ester) with full or partial hydrolysis under acidic or basic conditions (see Fig. 3). The products of the basic hydrolysis tend to be yellow and those of the acidic hydrolysis are mainly colorless.
Figure 3. Synthesis of poly(vinyl alcohol) (PVAl) with partial hydrolysis of poly(acetic vinyl ester) (PVAc).
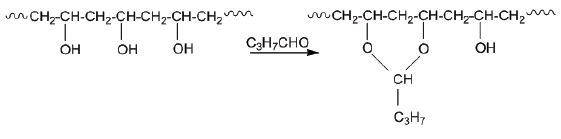
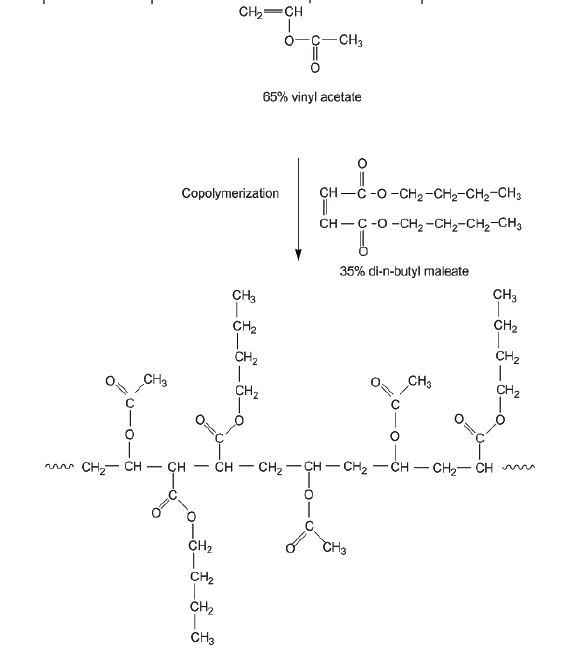
Poly(vinyl butyral) (PVB) is produced from the reaction of poly(vinyl alcohol) with butyraldehyde and is found on the market as Mowithal or Rhovinal (see Fig. 4). Poly(vinyl alcohol) is used for the consolidation of archaeological textiles and is produced by the polycondensation reaction from monomers of ethylene glycol. In Figure 5, the synthesis of Mowilith DMC2 is shown from the copolymerization of 65 % acetic vinyl ester and 35 % maleic di-n-butyl ester.
Figure 4. Synthesis of poly(vinyl butyral) (PVB) from the reaction of poly(vinyl alcohol) with butyraldehyde.
Figure 5. Synthesis of Mowilith DMC2 (65 % vinyl acetate and 35 % di-n-butyl maleate).
6 Deterioration of the Polymeric Films with Time
The aging of a polymeric film depends on:
- The chemical structure.
- The presence of additives (plasticizers, antioxidants, UV/Vis stabilizers).
- The method of application on the textile.
- Environmental factors.
- The conditions of exposure upon exhibition and storage.
Because of the physical and chemical processes of aging, many of the properties of the polymeric film change, such as the color, adhesion, resistance to tension, flexibility, and collection of dust. Additionally, the textile can shrink or even be torn. The aging of a polymeric film is a result of two different changes: The aging that comes from the decomposition of the polymeric chain and the aging that comes from the migration of the different additives.
6.1 Aging of the Polymeric Chain
Free radicals can be present in polymers produced by free-radical polymerization and some remaining carbon–carbon double bonds. That makes these materials vulnerable to oxidation and other chemical processes. The presence of quaternary carbon atoms enables the polymer chains to break and undergo depolymerization due to the low strength of the monomer–monomer bond. In polymethacrylic esters, like poly(butyl methacrylate) (PBMA), chain breaking can occur upon thermal decomposition (see Fig. 6).
Figure 6. Thermal degradation of poly(butyl methacrylate) (PBMA).
Chain breaking can be the result of a photo- or thermal oxidation as a random event or as a process of depolymerization starting from the chain endings and moving from section to section. A result of the depolymerization is a reduction of the mechanical properties. The ratio of the amorphous to crystalline areas changes, which causes more cracking and greater rigidity and brittleness (see Fig. 7).
Figure 7. Oxidation of polyethylene glycol (PEG).
During aging experiments, under accelerating conditions, an increase in the weight of the polymeric films was observed. This is probably caused by the consumption of oxygen during the photo- and thermal oxidation and as a result changes in the color and solubility occur. Additionally, a loss of weight can be observed with the removal of the side groups from the polymers. The removal of the acetyl side groups and hydrogen atoms leads to sequences of double bonds, which causes the yellowing of the remaining polymer (see Fig. 8) due to the creation of high conjugation.
The color of the polymeric chain is yellow or yellow/brown owing to the presence of unsaturated chromophore groups. A result of the removal of polar side groups, the closeness of the macromolecular chains increases the crystallinity, fragility, and brittleness. The solubility of the polymer changes significantly due to the reduced number of polar side groups of the carbon chain.
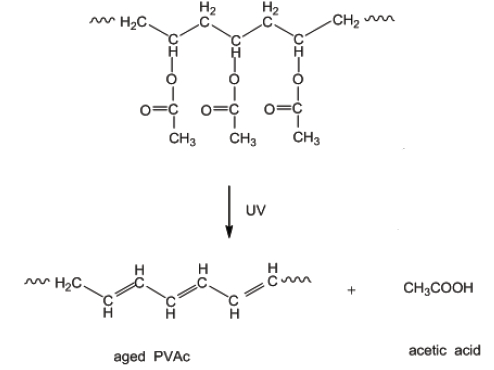
Figure 8. Removal of the acetyl side groups and hydrogen atoms (acetic acid) from polyvinyl acetate (PVAc).
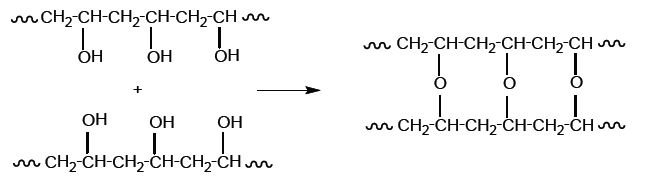
Cross-linking is another type of chemical decomposition that occurs in polymers. The creation of cross-linked bonds in polymers is often developed with photo- and thermochemical free-radical reactions and is usually accelerated by acidic impurities and metal compounds that are present from the dyes of the textiles.
Increased rigidity and toughness of the Paraloid F10 film (a thermoplastic acrylic resin) is attributed to its capability to form cross-linked bonds. Poly(vinyl alcohol) is known for its ability to form cross-linked bonds (see Fig. 2). The cross-linking reaction takes place from the side hydroxyl groups of the two polymers. Yellowing and chain breakage of PVAl has been observed at 80 °C, with cross-linking occurring at 100 °C, which results in the reduction of solubility.
6.2 Aging Due to the Migration of Additives
Antioxidants are added to reduce the sensitivity of the synthetic polymers to photooxidation and the oxidation caused by heat. However, if low-molecular-weight antioxidants like butylated hydroxytoluene (BHT) in polyethylene are used, they might migrate to the surface of the polymer, leaving the material sensitive to oxidation. Additionally, BHT reacts very easily with nitrogen oxides in the atmosphere, thereby forming a yellow product on the textile. Kenneth and Smeltz [8] attribute the yellowing of white textiles to this process and to the polyethylene bags in which the textiles were stored.
Almost all synthetic polymers contain low-molecular-weight plasticizers. The producers are not required to inform consumers of plasticizer contents below 5 % in synthetic polymers. The migration of 3–5 % of the plasticizer does not significantly change the flexibility of the material and usually does not create serious problems with aging.
However, if they are present in higher amounts, the migration can cause significant changes in the appearance of the material. These include the coloration of the polymer surface and the transformation to a sticky surface, vulnerable to collecting dust. Plasticizers, which are usually esters from organic acids, on reaction with atmospheric humidity, can cause corrosion of the metals and coloration and degradation of the organic materials.
Conclusion
There are numerous factors that can cause the degradation of textiles. That is why the protection and good conservation of textiles are necessary. A huge variety of products is available on the market. Each product (adhesive or consolidant) that is applied exhibits advantages and disadvantages.
With the rapid development of science and technology in recent years, the properties of these products are improving. In each case, the protection/conservation process that is chosen for each work of art should be reversible and incapable of creating an even worse situation, so that the textile can safely exist for future generations.
References
[1] L. K. Herrera, A. Justo, A. Duran, M. C. Jimenez de Haro, M. L. Franquelo, J. L. Perez Rodriguez, Identification of cellulose fibres belonging to Spanish cultural heritage using synchrotron high-resolution X-ray diffraction, Appl. Phys. A 2010, 99, 391–398. https://doi.org/10.1007/s00339-010-5626-z
[2] Tatiana Koussoulou, Photodegradation and photostabilization of historic silks in the museum environment-evaluation of a new conservation treatment, Papers Inst. Archaeol.1999, 10, 75–88. https://doi.org/10.5334/pia.135
[3] G. S. Egerton, The mechanism of the Photochemical Degradation of Textile Materials, Coleration Technol. 1949, 65(12), 764–780. https://doi.org/10.1111/j.1478-4408.1949.tb02558.x
[4] Rosario Lopez Cisneros, Abel Gutarra Espinoza, Marta I. Litter, Photodegradation of an azo dye of the textile industry, Chemosphere 2002, 48(4), 393–399. https://doi.org/10.1016/S0045-6535(02)00117-0
[5] Brojeswari Das, A. Dac, V. K. Kothari, R. Frangmiro, M. de Araujo, Moisture transmission through textiles, Part II, Evaluation methods and mathematical modeling, AUTEX Res. J. 2007, 7(2), 100–110. http://www.autexrj.org/No2-2007/0236.pdf
[6] D. W. Grattan, M. Bilz, The thermal aging of Parylene and the effect of antioxidant, Studies in Conservation 1991, 36(1), 44–52. https://doi.org/10.1179/sic.1991.36.1.44
[7] Agnes Timar Balazsy, Dinah Eastop, Chemical Principles of Textile Conservation, Routledge, Oxford, UK, 1998. ISBN-10: 0750626208
[8] C. Kenneth E. I. Smeltz, The yellowing of white fabrics and garments stored in polyethylene bags, National Technical Conference AATCC (American Association of Textile Chemists and Colorists) 1982, 137–143.
The article has been published in Greek as:
- Εφαρμογή των συνθετικών πολυμερών – στη συντήρηση ιστορικών υφασμάτων,
Evrykleia G. Karagiannidou (Ευρύκλεια Γ. Καραγιαννίδου),
Chemica Chronica (Association of Greek Chemists Magazine) 2016, 78 (7), 19–24.
Link to Chemica Chronica
and was translated by Evrykleia G. Karagiannidou.
Author
Evrykleia G. Karagiannidou studied chemistry and gained her MSc in Polymer Chemistry and Technology from Aristotle University of Thessaloniki, Greek. Since 2006, she is a research scientist in the Analytical Development Laboratory of Pharmathen, Athens, Greece.
She has been occupied with the conservation of archaeological artifacts such as marble, stone, textile, ceramic, glass, and wall painting, and has published many research papers in this field.

.JPG)