How can a tiny molecule like ethanol be at the root of so much human misery?
Here we propose to get to the bottom of the chemical consequences of a night of celebrating to excess.
3 Ethanol Leaves — and a Hangover Arrives
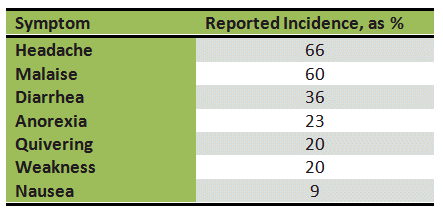
With regard to effects of ethanol on physical and mental performance, the intoxication phase is what usually occupies center stage. But physiological changes don’t end with the return to a normal blood-alcohol concentration. That is the point at which the hangover begins, with its many unpleasant consequences (see Tab. 2) [17].
Though it is common simply to smile at the notion of a hangover, which in most cases proves to be self-healing, this nevertheless represents a health condition to take seriously, and one with major economic consequences. Numerous studies in various industrialized countries have shown that hangovers result in economic damage on the order of several hundred euros per person and year. Apart from absenteeism, the highest of the costs stem from — mostly unnoticed — losses in productivity. Studies with pilots, motorists, and skiers show that performance is greatly reduced during a hangover, and the risk of accidents increases.
Table 2. Relative frequency of various hangover symptoms.
It is surprising that, despite its economic significance, the mechanisms and physiological causes of the hangover have never been a focus of serious scientific interest. Perhaps this is because many — and not merely victims, but also scientists — believe that a hangover is a legitimate and deserved punishment for senseless, exorbitant drinking, and that a self-induced and self-healing disease doesn’t warrant the effort and expense associated with a thorough scientific investigation.
Before we unravel the mystery of the hangover from a chemical standpoint, we should recognize that a bacchanalian revel leads to no place other than a serious case of ethanol poisoning, which in turn produces significant disruptions and changes in a number of metabolic processes throughout the body. This explains why different people perceive hangovers so differently. Even a single individual may, after ingestion of a given amount of alcohol, experience both the intoxicating effects and their repercussions entirely differently on different days.
4 Biochemistry of the Hangover
One part of the secret associated with the hangover derives from the fact that the syndrome itself is poorly defined in terms of physiological measurement. Consequently there are no objective bases for evaluating the seriousness of the condition, because subjectively the symptoms are perceived differently, with a very wide range of variation. In what follows, possible biochemical sources of some of these symptoms are considered.
4.1 The Symptoms
Dehydration
To begin with, alcohol has a diuretic effect, which is to say that it causes an increase in the production and excretion of urine.
After drinking about three shots of 100 proof spirits (ca. 50 g of ethanol in 120 mL of liquid), one would eliminate in the hours that follow between 600 and 1000 mL of water (roughly 1.5 to 2 pints).
Ethanol decreases release of the antidiuretic hormone vasopressin from the posterior pituitary, which reduces reabsorption of water in the kidneys, so more urine is excreted.
Vomiting, sweating, and diarrhea also increase the loss of fluids and electrolytes, leading to dryness of the mouth, thirst, and dizziness, all typical hangover symptoms. This corresponds to clinical experience showing that water intake during a period of hangover can relieve these particular symptoms.
Hypoglycemia and Acidosis
A drop in the NAD+/NADH ratio has far-reaching consequences for metabolism in general. It leads to an accumulation of lactic acid (acidosis), and lowered production of glucose (gluconeogenesis), i.e., the glucose concentration in the blood declines. Overall, the resulting hypoglycemia causes general bodily weakness as well as mood changes; also typical hangover symptoms.
Fructose is metabolized in the body to glyceraldehyde, which is then reduced by NADH to glycerin. This produces NAD+, which could in principle raise a depressed NAD+/NADH ratio. Unfortunately, the fructose intake required is far too great for such an effect ordinarily to be perceptible. An attempt some years ago in British pubs to sell fructose tablets as a “sobering agent” for use after a round of carousing had to be abandoned for this reason on account of demonstrable ineffectiveness.
Disturbances in the Gastrointestinal Tract
Alcoholic beverages with low alcohol content (e.g., aperitifs) stimulate the production of stomach acid. Hard drinks with greater than 20 vol % alcohol irritate the stomach lining, leading to inflammation (gastritis). Moreover, pancreatic secretion is stimulated, and intestinal activity increased. These effects taken together can contribute to the stomach and abdominal pains, nausea, and vomiting typical of a hangover.
Sleep Disorders and Circadian Rhythm Alteration
Although alcohol has a sedative effect, and causes one to become sleepy, sleep that follows inebriation tends to be of short duration, and offers little restorative value. The 24-hour rhythm of body temperature is also affected, since one’s temperature is reduced during the period of inebriation and increased in a hangover. Moreover, overall hormone production by the pituitary gland is altered, so one’s day-night rhythm becomes discombobulated, and a hangover might even be viewed as analogous to “jet lag”.
Headaches
Alcohol causes expansion of the blood vessels, and this can cause a headache. Alcohol also influences the production and effectiveness of numerous compounds in the central nervous system, including neurotransmitters, histamine, serotonin, and prostaglandins. All this could also contribute to headaches, although the true direct source of the relationship between alcohol consumption and headaches remains in doubt.
4.2 The Culprits
Alcohol Withdrawal
Since some hangover symptoms resemble signs of withdrawal suffered by alcoholics, it might be that a hangover could be regarded as an early, mild form of a withdrawal effect. This hypothesis is in fact widespread, but the two conditions can be sharply differentiated.
Many hangover symptoms, such as diminished brain wave activity, absence of increased blood pressure, dehydration, nausea, and dry mouth are seldom observed during alcohol withdrawal in the case of chronic drinkers. Some of the physiological changes (cytokine and thromboxane production) resemble those of a viral infection, and indeed the symptoms of flu are similar to those of a hangover.
Acetaldehyde
The acetaldehyde resulting from oxidation of ethanol is highly reactive and toxic, and it reacts with numerous metabolic products. In most people, acetaldehyde is decomposed so quickly that, although in the wake of inebriation there is indeed an increase in the blood concentration of acetaldehyde, it always remains under 2 µg/L. In a fraction of the oriental population, however, the acetaldehyde concentration after drinking rises quickly to a twenty-fold value, and this in turn triggers drastic hangover symptoms, so some researchers have come to regard acetaldehyde as the true culprit in inducing hangovers.
Methanol and Fusel Oil
Beer, wine, and spirits are not pure ethanol/water mixtures, but instead contain — depending on the method of production — thousands of other compounds.
In a classic study, G. K. S. Pawan from Middlesex Hospital, London, UK, investigated various drinks in terms of their tendency to produce a hangover [18]. Twenty healthy, male volunteers (no alcoholics) got drunk at weekly intervals successively on red wine, white wine, rum, whiskey, gin, vodka, brandy, or straight alcohol (20 vol % in water). The amount consumed was always measured such that each subject reached a blood-alcohol content of 1.7 ‰ – in other words, complete intoxication.
After eight weeks, each participant had experienced and survived a fully intoxicated state from each type of drink, and was able to evaluate the severity of the resulting hangover symptoms. It turned out that there was a definite relationship between the intensity of the hangover and the beverage: brandy produced the most severe hangover, followed (in descending order) by red wine, rum, whiskey, white wine, and gin. Vodka and pure alcohol were tolerated the best.
Fusel Oil
It is well known that higher alcohols present in relatively high concentration in beer, wine, and spirits all can cause hangover symptoms. The fraction boiling above 95 °C (ethanol b.p. = 78 °C) is designated as fusel oil. This may be shown to contain up to 50 different components, where the chief constituents are isobutanol (2-methyl-1-propanol), propanol, and above all, the pair of isoamylalkohols: 2-methyl-1-butanol and 3-methyl-1-butanol. The latter two form from the amino acids isoleucine and leucine by protein degradation during fermentation (Fig. 5).
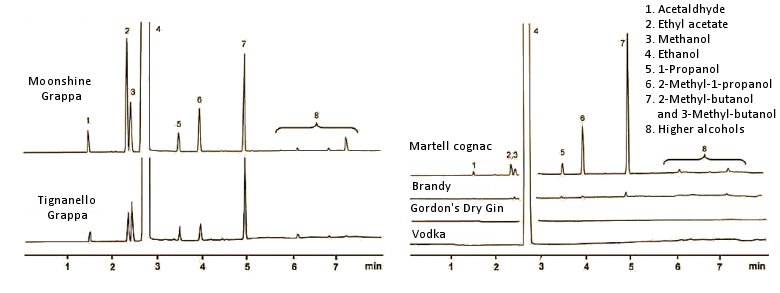
Figure 5. Gas chromatograms of some spirits. In each case, 1 μL of the spirit was introduced into a helium stream passing through a 25 m column (0.32 mm diameter), with a coating of polyethylene glycol (molecular weight 20,000). Polyethylene glycol is a relatively polar material with a great affinity for other polar compounds, so small nonpolar molecules pass through the column most rapidly. Each gas chromatographic signal represents at least one substance. The signal for ethanol, present in great excess, has been truncated.
Right: Clear grain spirits, like vodka and gin, contain — apart from ethanol — only small amounts of other volatile compounds. Brandy contains only ca. 10 % wine distillate, and is brought up to 28–32 vol % by the addition of grain alcohol. A pure brandy, such as cognac, contains a multitude of volatile compounds, which contribute significantly to its typical bouquet and taste. In other words, in the absence of these admixtures, cognac would no longer be cognac.
Left: The gas chromatograms of two grappas reveal something about their origins. Grappa is prepared by the fermentation of grape residues after pressing, with subsequent distillation. As a fermented and distilled grape product, grappa contains volatile ingredients also typical of cognac. Compared with the premium grappa from Marchesi Antinori, Florence, Italy, the grappa distillate from a rural “moonshiner” (above) displays a significantly larger fusel oil fraction. Especially noteworthy is the increased ethyl acetate content (signal 2), which lends grappa a relatively sharp tone. Moreover, the increased level of long-chain, higher-boiling alcohols points to an unprofessional distillation that was terminated much too late, since fusel oils distill over precisely in the tailings. This grappa actually does taste unpleasantly sharp, and has a penetrating aftertaste, strongly reminiscent of solvent (ethyl acetate).
Methanol
Methanol is the most toxic of the aliphatic alcohols, and the one of greatest toxicological interest. Its oxidation leads to formaldehyde and formic acid. Typical symptoms of methanol poisoning are visual disorders (e.g., the impression of a snowstorm), which can lead to blindness and even death. In the case of serious poisoning, one can actually smell formaldehyde on the subject’s exhaled breath.
Methanol has a lower affinity for ADH (alcohol dehydrogenase) than ethanol, which is to say that with a mixture of the two alcohols, it is the ethanol that is metabolized first [19]. Drinking a large quantity of methanol-containing spirits can therefore lead to an accumulation of methanol, which is itself affected only after complete metabolism of the ethanol. On the other hand, in the case of acute methanol poisoning, ethanol is often introduced into the victim intravenously in order to block the oxidation of methanol and gain time: for dialysis, for example.
Almost all spirits contain some methanol, which arises as part of the fermentation process from pectins. Pectins are linear polysaccharides derived from galactouronic acid units, where some of the carboxylic acid groups are esterified with methanol (see Fig. 6).
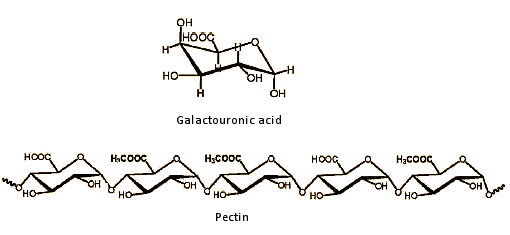
Figure 6. Pectin.
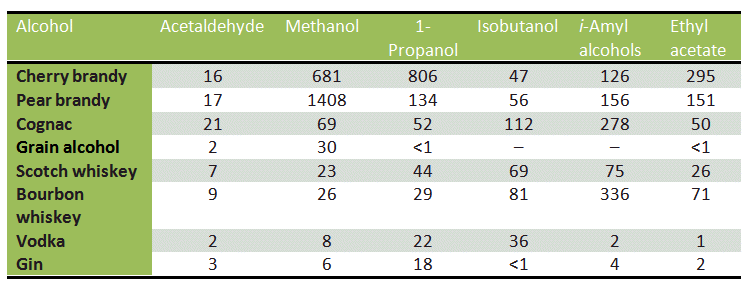
During fermentation, the ester groups are hydrolyzed, and methanol is released. An especially high methanol concentration is associated with the brandies derived from various types of fruit, such as pears, plums, and cherries, which contain large amounts of heavily esterified pectin (see Tab. 3 and Fig. 5).
Table 3. Volatile compounds in spirits [34]. All reported quantities in mg/100 mL of pure alcohol; i-amyl alcohols are 2-methyl-butanol and 3-methylbutanol.
5 Taming a Hangover
Boozy celebrations can lead to hangovers taking a variety of forms, and tormenting the body in every place imaginable, often in subtle ways. But the multitude of possible metabolic disruptions is far outnumbered by the inventory of traditional recipes and quack remedies for “curing” a hangover. Indeed, what HASN’T been tried for hangover relief? Leg compresses, every conceivable sort of tea and herb extract, eating pickled herring and dill pickles (in fact, one would be strongly advised to avoid pickled herring and dill pickles, a proverbial hangover breakfast, since an assaulted stomach lining absolutely cannot tolerate acidic and aggressive foods), warm milk with honey, further imbibing in the last alcoholic beverage consumed, athletics, vitamin tablets, dextrose, juice, chicken broth, etc., etc. Almost none of these therapeutic measures have been subjected to clinical testing, which many would regard as unnecessary anyway since all of them help, sooner or later. Finally, almost every hangover ends eventually, with or without treatment.
Some of the hangover symptoms can be alleviated medicinally, for example by acidic buffers in the case of nausea and gastritis. Aspirin and ibuprofen will help with headaches, but they simultaneously induce the stomach to generate acid, which should be taken into consideration in the case of upper abdominal pain and nausea.
Acetaminophen (paracetamol) is a popular remedy for headaches, and is present in many over-the-counter painkillers. But frequent ethanol consumption activates MEOS metabolism (Microsomal Ethanol Oxidizing System), and resulting monooxygenases can oxidize acetaminophen to the carcinogen N-acetyl-p-benzoquinone imine [20]. It is true that MEOS ethanol metabolism ordinarily plays only a secondary role, but acetaminophen still should not be taken to combat a hangover.
A great many other things have been tried. The alleged positive effects of beta-blockers, serotonin antagonists, and coffee consumption could not be verified clinically [21]. With a stomach under attack, one should in any case forego coffee.
Overall it must be soberly reported that a hangover is comprised of many factors, and that little can be done for it therapeutically: There is no real cure for a hangover. ChemViews recommends lots of water against the dehydration, aspirin or ibuprofen against the throbbing headache, fruit juice against hypoglycemia, Mom’s powerful chicken soup to compensate for electrolyte losses, a vitamin pill because of its powerful placebo-effect, compassion and words of comfort from loved ones, and finally — if blood circulation and control of the lower extremities permit an upright walk — a long stroll in fresh air. Meanwhile, one should think deeply about the pointlessness of excessive drinking, because only one thing is absolutely certain: one only gets a hangover only after a bout of heavy drinking.
All this helps and on the next day it will all be over — at least until next time. Well then: Cheers and Bottoms up!
-
Chemistry of a Hangover — Alcohol and its Consequences Part 1
-
Chemistry of a Hangover — Alcohol and its Consequences Part 2
References
[17] J. G. Wiese et al., Ann. Int. Med. 2000, 132, 897. Link
[18] G. L. S. Pawan, Proc. Nutr. Soc. 1973, 32, 15A. DOI: 10.1079/PNS19730007
[19] H.-T. Haffner et al., Legal Medicine 1992, 105, 111.
[20] C. Girre et al., Alcoholism: Clin. Exp. Research 1993, 17, 170. DOI: 10.1111/j.1530-0277.1993.tb00743.x
[21] R. Swift, D. Davidson, Alcohol Health & Res. World 1998, 22, 54. Link
Author
Prof. Klaus Roth, Freie Universität Berlin, Germany.
The article has been published in German in:
and was translated by W. E. Russey.
Other articles by Klaus Roth published by ChemistryViews magazine:
- In Espresso — A Three-Step Preparation
Klaus Roth proves that no culinary masterpiece can be achieved without a basic knowledge of chemistry
DOI: 10.1002/chemv.201000003 - In Chocolate — The Noblest Polymorphism
Klaus Roth proves only chemistry is able to produce such a celestial pleasure
DOI: 10.1002/chemv.201000021 - In Sparkling Wine, Champagne & Co
Klaus Roth shows that only chemistry can be this tingling
DOI: 10.1002/chemv.201000047 - In The Chemist’s Fear of the Fugu
Klaus Roth shows the chemist’s fear of the fugu or pufferfish extends as far as the distinctive and intriguing poision it carries
DOI: 10.1002/chemv.201000104





This is a complicated subject and although this article has a lot of science and chemistry it has a few basic mistakes. First of all, we need to separate the effects of ethanol while drinking, and the effects AFTER drinking. It is nice to know what happens to our bodies when we drink but the subject is HANGOVER which happens AFTER drinking. First, we have to break down the issue into effects while drinking- primarily from binge drinking, and the effects that happen later. No one can deny that if you consume a lot of alcohol in a short time you will have a bad reaction. The body is going into preservation mode. That to me is not a hang over. That is being drunk. So it the speech issues and walking and other things mentioned.
The article refers to certain % blood level so let’s assume that a person has “moderate” consumption of one drink every 30 or 45 minutes over maybe 5 to 7 hours. Typical for a party or a day of football watching. During that consumption a person is not likely to suffer any horrible effects. For sure they don’t need to drive or anything like that, but I am going to talk about the hang over…..that happens later. I am not sure the blood alcohol levels between a good buzz and very drunk and vomiting, but let’s assume it is .8 to 1.0. One trouble with the article is it shows some of the other chemicals that are present in most fermented beverages, but really only refers to the effects of ethanol. I am pretty sure that acetaldehyde, methanol, and the other alcohols present also have some effect. I understand the challenge since many beverages have many chemicals and it would be difficult to test the effects of some of them on people. BUT what we know is that methanol in concentrated form is very nasty. It will cause blindness and death if taken in large doses. And now to my main point. As a distiller the hang over is a major concern. And what I can say without a doubt is that the main symptoms of a hang over are caused by methanol. To clarify when I say hang over I mean the symptoms that occur LATER after the drinking is done (assuming no binging). Mainly it is a throbbing headache and malaise and generally feeling as if you have been run over by a truck. In this case these symptoms occur if there is moderate over consumption (say over .8 blood alcohol level) or totally @#$% faced. Here is what happens. Your liver will metabolize the ethanol first and will not metabolize methanol until all of the ethanol is out of your body. Then the liver starts on methanol and metabolizes it into formaldehyde and finally formic acid. Not good stuff. The formaldehyde reduces blood flow to the brain which most likely causes the throbbing head. Think about it. When do you feel the “hang over”? Not while drinking, but later.
If you want to know how I know that methanol causes the hang over, it is because I am a distiller and through distillation we can remove the methanol and the other alcohols and many of the so called “fusel oils” (they are not really oils). But I can drink the “tails” from the still (not great tasting) but they do not cause a hang over. With the methanol taken out we never have a hang over either. One of the main issues with most studies is the use of BEER and WINE as the alcohol. The fact is all beer and wine have methanol in them! If you really want to do a test use an alcohol that has the methanol removed (mostly). Brown spirits get a bad rap for hang over. It is NOT the browness!! It is NOT tannins. It is methanol. Here is the fact. Some clear spirits such as vodka and gin MUST be distilled to over 190 proof (95% pure ethanol know as a neutral spirit because ethanol has no taste or aroma). The table in the article clearly shows a lower amount of methanol in vodka and gin. Also rum MAY be distilled to 190 proof so some types of rum (generally clear) will have lower amounts of methanol. If you do not MIX a vodka or gin with other alcohol you will generally have no or less hang over. (With distillation it is possible to reach 195 proof or 97.5% purity so it is possible that some vodkas and gins can vary slightly in the amount of methanol present and have more or less hang over effect, but generally less that some other products). Brown spirits- namely whiskey- MAY NOT be distilled over 160 proof (80%). They therefore have more “fusel oils” which would include methanol. (Fusel oils and “congeners” are generic terms used to describe the chemicals other than water and ethanol present in beverages.) One issue is most drinkers who are at a party or event do not drink ONE product for the entire time. In that case it is impossible to determine what the culprit is if you have a hang over. I challenge anyone to drink a distilled spirit with most of the methanol removed and see if you have a hang over. You will get the buzz and can certainly over do it and have a bad experience, but you will not get the nasty effects of the hang over. The true scientist will do their own experiments to determine the validity of the experiment. Because we are all different the AMOUNT you consume needs to be “standard” for you. Let’s say you have six ounces of one product and you get a hang over. Then try 6 ounces of a different product and see what happens. As the table shows, different things have different quantities of methanol and will have a different effect. (Methanol is fermented from the “woody” substances in the fermented material. It is also known as “wood alcohol”)
One last thing…I want to put to bed all of the hang over myths. It is TRUE that ethanol is a diuretic. You will urinate more. Coffee and tea are also diuretics and I never get a hang over from them. Drinking a glass of water between every alcoholic beverage only serves to SLOW YOU DOWN in consumption and thus reducing the outcome. It is wise to stay hydrated, but it does not prevent a hang over. Nor does eating, or sleeping, or taking electrolytes, or any of the popular “remedies.” If we really want to get scientific on this subject we need to test products that do not have methanol (or very small amounts) and all of the other “fusel oils” present to see what happens. It is a simple test. One last thing…there are two antidotes to methanol poisoning. One is from the hospital and the other is likely in your house. Ethanol is an antidote! Yes, the hair of the dog. BUT NOT THE DOG THAT BIT YOU!! The one that bit you has methanol and drinking more of it will just postpone the misery. If you do not have a beverage without methanol then the best option is VODKA or GIN. Remember how they MUST be distilled and they tend to be mostly ethanol. The good old Bloody Mary is actually effective. The best option though is to drink beverages with little or no methanol. If you get a hang over you drank methanol. Period. If you disagree then let’s put it to the test.