Combustion Processes
- Melting of the fuel
- Transport of the fuel by the capillary action of the wick
- Conversion of the liquid fuel into gas
- Thermal decomposition (pyrolysis) of the fuel
- Oxidation of the pyrolysis products
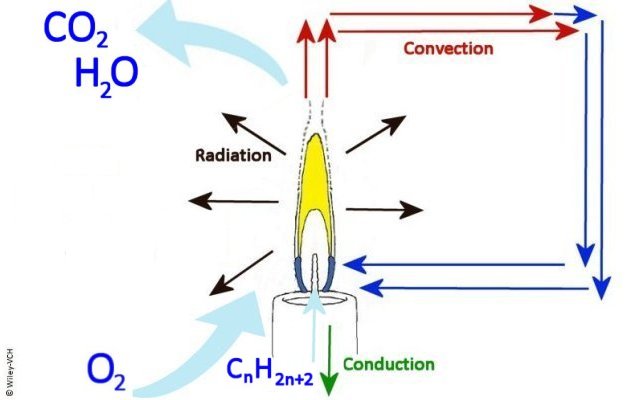
Solid carbon particles, soot, form at about 1,000 °C. These are excellent blackbody radiators of colors in the yellow to red spectrum. The typical yellow color of a candle flame or wood fire is therefore produced primarily by the hot soot. The mixing of the fuel and the O2 is the slowest part of the combustion process and, therefore, the rate determining step.
Zones of a Laminar Diffusion Flame
Heat and Mass Transfer Pathways
See also:
- Chemistry of the Christmas Candle — Part 1
When we light a candle, the chemistry we are pursuing is not only especially beautiful, but also especially complex - Chemistry of the Christmas Candle — Part 2
We pursue the fate of a single wax molecule in a burning candle - All Clever Pictures
.gif)




How about olive oil flame, what makes it? is virgin olive oil flame looks like a non virgin olive oil one?