Intermolecular reactions are triggered by the collision of reaction component molecules. The efficiency is essentially dependent upon the homogeneity of the reaction solution. Several mixing techniques have been established to achieve such homogeneity and realize effective intermolecular reactions.
Electrochemical approaches, for example, have been employed to regulate chemical transformations at the surfaces of electrodes. From the synthetic aspect, an electrochemical approach can be used to generate transient intermediates such as ions, radicals, or radical ions through electron transfer at the electrodes to afford not only functional group transformations, but also carbon–carbon bond-formation reactions.
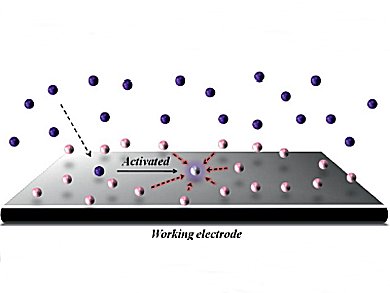
Kazuhiro Chiba and colleagues, Tokyo University of Agriculture and Technology, Japan, have demonstrated the concept of surface-condensed electrodes that enables efficient intermolecular carbon–carbon bond-formation reactions. Reaction partners were noncovalently condensed at the electrode surfaces. These could effectively trap transient radical cation intermediates generated at the electrodes to offer opportunities for rapid intermolecular reactions.
The concept of surface-condensed electrodes would be a significant aid for directing intermolecular reactions.
- Efficient Intermolecular Carbon–Carbon Bond-Formation Reactions Assisted by Surface-Condensed Electrodes,
Yohei Okada, Yusuke Yamaguchi, Kazuhiro Chiba,
Eur. J. Org. Chem. 2011.
DOI: 10.1002/ejoc.201101313