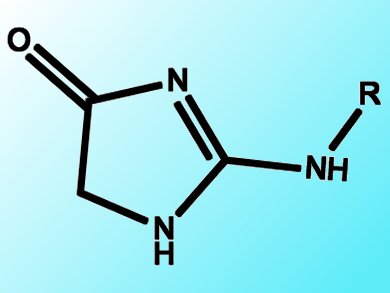
Hydantoins are an important class of compounds, as they can be used as building blocks for the synthesis of therapeutic drugs. The hydantoin core structure can be found in drugs associated with the treatment of epileptic seizures, metastatic prostate cancer, and cardiac arrhythmias. Moreover, the hydantoin scaffold is a very attractive template for combinatorial synthesis due to its large number of possible substitution patterns and interesting combination of potential hydrogen-bond donors and acceptors on the small five-member ring system. Therefore, the efficient synthesis of a diverse library of hydantoins is attractive.
A Korean team headed by Ge Hyeong Lee, Korea Research Institute of Chemical Technology, Daejeon, has reported a new protocol for the synthesis of a diverse aminohydantoin library starting from a variety of amino amides and isocyanates or isothiocyanates. Intramolecular cyclization of thioureas or ureas tethered to amides afforded the aminohydantoins in very high yields regardless of the substituent (alkyl or aryl).
This method is attractive because the starting materials are readily available and the reaction can be performed under mild conditions. Importantly, the chiral centers remain intact under the reaction conditions, which makes this method even more valuable.
- Diversified Aminohydantoins from Ureas and Thioureas Tethered to Amides,
Sukumar Bepary, In Kwon Youn, Hee-Jong Lim, Ge Hyeong Lee,
Eur. J. Org. Chem. 2012, 13, 2542–2548.
DOI: 10.1002/ejoc.201200025