Electrical energy storage is currently attracting intense attention, as the design and fabrication of new energy-storage devices is likely to be instrumental in addressing global concerns regarding energy shortages and related environmental issues. Although lithium-ion batteries have been shown to exhibit long cycle lifetimes and high energy densities, the limited worldwide lithium resources is a cause for concern.
Yu-Guo Guo and Xiaosi Zhou, Chinese Academy of Sciences, Beijing, China, have turned their attention to sodium ions as an alternative to lithium ions. Sodium ions are approximately 55 % larger than lithium ions, therefore, their focus has been on developing a suitable material to host these larger alternatives.
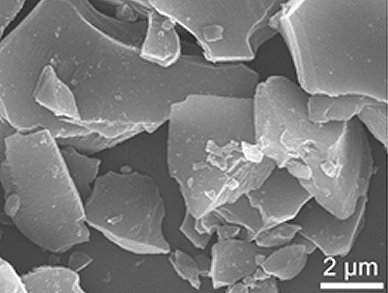
Highly disordered carbon was produced by using a simple self-assembly approach that was based on the electrostatic interactions between two oppositely charge polyelectrolytes in water. The resultant carbon material has an interlayer spacing of 0.39 nm between the graphitic layers, thus allowing for the desired insertion/extraction of sodium ions. In addition, when used as an anode material for sodium-ion batteries, superior cycling stability and rate capabilities were observed.
This highly disordered carbon anode represents a promising design for new electrochemical-storage devices that utilize larger but more abundant sodium or magnesium ions.
 Highly Disordered Carbon as a Superior Anode Material for Room-Temperature Sodium-Ion Batteries
Highly Disordered Carbon as a Superior Anode Material for Room-Temperature Sodium-Ion Batteries
Xiaosi Zhou, Yu-Guo Guo,
ChemElectroChem 2013.
DOI: 10.1002/celc.201300071
ChemPubSoc Europe’s new journal ChemElectroChem is dedicated to covering the entire scope of pure and applied electrochemistry.