Plasmonic Polymers
Molecules can copolymerize to form longer composite chains; it turns out that nanoparticles called colloidal particles can also copolymerize to make hybrid nanostructures. The fact that these reactions occur in a very similar manner is not obvious, but this could be used to carry out fundamental studies of copolymerization reactions. However, colloidal polymers are primarily useful for the development of highly complex nanosystems. In the journal Angewandte Chemie, a team of Chinese, Canadian, and American researchers has presented a report about the copolymerization of gold nanorods of various sizes as well as gold and palladium nanorods.
Polymers made of metal nanoparticles are particularly interesting because of their plasmons – quantized charge carrier density oscillations resulting from the collective excitation of free electrons to plasma oscillations. Long chains of metal nanoparticles known as plasmonic polymers display strong interactions between the plasmons of the individual building blocks. Their optical properties can be controlled by means of factors like the degree of polymerization, the size of the nanoparticles, or the distance between particles. Copolymer chains made from nanoparticles with different sizes, shapes, and compositions are even more interesting as they offer another degree of freedom in tuning the properties (and potentially, leading to new properties) of plasmonic polymers. Potential applications could include smaller computer chips, improved nanoantennas and sensors, and improved optical data processing.
Copolymerization of Gold and Palladium Nanorods
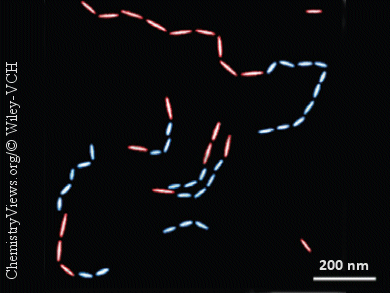
The researchers from Jilin University, China, the University of Toronto, Canada, and the University of North Carolina, USA, have now developed methods for applying strategies from molecular copolymerization (the polymerization of different monomers together) to the co-assembly of nanorods of varying sizes and composition. Led by Kun Liu and Eugenia Kumacheva, the team uses gold nanorods with polystyrene chains on the ends as building blocks. Addition of water to the organic solvent containing a suspension of the nanorods causes the polystyrene ends, which are only poorly soluble in water, to bond tightly together, connecting the nanorods into long polymer chains. This approach was extended to the co-assembly of random and block copolymers of gold nanorods of different length, as well as random copolymers of gold and palladium nanorods. (Random copolymers contain different monomers in a random order; in a block copolymer the polymer chain contains larger domains of either one or the other monomer.)
The researchers were able to establish a model for the reactions that confirmed and extended established kinetic theories for molecular stepwise copolymerization reactions. The colloidal polymers obtained also provide an excellent model system for the fundamental investigation of plasmonic properties, such as special modes resulting from the asymmetry of nanostructures with irregularly distributed components.
- Copolymerization of Metal Nanoparticles: A Route to Colloidal Plasmonic Copolymers,
Kun Liu, Ariella Lukach, Kota Sugikawa, Siyon Chung, Jemma Vickery, Heloise Therien-Aubin, Bai Yang, Michael Rubinstein, Eugenia Kumacheva,
Angew. Chem. Int. Ed. 2014.
DOI: 10.1002/anie.201309718
For an interview with Professor Eugenia Kumacheva see:
- Copolymerization of Metal Nanoparticles,
Jonathan Faiz,
ChemViews Magazine 2014.
DOI: 10.1002/chemv.201400002