4. How Do We Actually “Taste” Hotness?
When a bowl of chili con carne is described as “tasting good” because the level of spiciness (or “hotness”) is just right, strictly speaking this is incorrect, because we are actually only able to “taste” sweet, bitter, sour, salty, and umami. Hot/spicy isn’t on the list!
But why not? After all, we can perceive both spiciness and temperature with our tongues, as well as various more subtle aspects of flavor. The reason for a strict differentiation here has to do with neuronal processing: information about taste (flavor) is acquired through special sensory cells in the tongue, the responses of which are passed to the brain by way of three cranial nerves, Nervus facialis, N. glos sopharyngealis, and N. vagus, and the processing finally occurs in the brain. Messages related to excessive temperature or spiciness come through yet another cranial nerve, the Nervus trigeminus. Fine branches of the Trigeminus permeate, among other places, the mouth, including the tongue. Nociceptors, very special receptors, are found at the free ends of the Trigeminus.
4.1 Nociceptor
The term “nociceptor” is derived from two words, nocere (Latin for pain) and receptere (latin for receive), so these receptors are sometimes simply called “pain receptors”. This term is not really accurate, however, because the electrical signals generated must first be further processed in the brain before they can be consciously associated with the sensory perception “pain”. But how “pain” is actually perceived depends to a great extent upon other factors, which Fein [31] suggests can be envisioned as a “black box” for transforming a stimulus into electrical impulses. Birth pain, for example, is often not perceived as painful, but rather as blissful – because of the nature of its origin – in contrast to the slightest pain that might arise during a minor dental procedure, which at the time can seem absolutely unbearable.
Irrespective of whether the tongue comes in contact with a soup that is far too hot, or with spicy cayenne pepper, signals generated by nociceptors in the tongue (at least above a certain level) produce essentially identical sensory impressions of “pain” [32].
For us this should not really come as a surprise: we already know from (unpleasant) experience that we can “burn” our tongues  either with food that is too hot (in the sense of temperature), or something which is too spicy/hot. Nevertheless, there remains one small – but significant – difference. Eating a soup that is too hot leads to pain that can persist for a long time, and we are acutely aware of the resulting tissue damage to the tongue for days after. On the other hand, a pinch of cayenne pepper may, in the very same way, “hurt like hell” at the moment it is eaten, but the pain will subside within a few minutes, leaving no lingering after effects.
either with food that is too hot (in the sense of temperature), or something which is too spicy/hot. Nevertheless, there remains one small – but significant – difference. Eating a soup that is too hot leads to pain that can persist for a long time, and we are acutely aware of the resulting tissue damage to the tongue for days after. On the other hand, a pinch of cayenne pepper may, in the very same way, “hurt like hell” at the moment it is eaten, but the pain will subside within a few minutes, leaving no lingering after effects.
4.2 TRPV1
Thanks to numerous ingenious studies, we are now in a position to see through this example of chemical deception. Embedded in cell membranes is another type of receptor, called “TRPV1” (transient receptor potential vanilloid subfamily 1). This protein acts as a heat sensor [33–36]. TRPV1 is essentially a switchable ion channel activated by high temperature. Its normal switching point lies above 42 °C (108 °F), but the binding of a ligand, such as capsaicin, can cause this to be lowered to a value below standard body temperature (37 °C) [37]. In the case of capsaicin, this results in constant activation, and thus transmission of a continuous sensation of pain.
When the true temperature at a TRPV1 receptor exceeds 43 °C [31], the receptor is of course activated as well, causing it to open a channel through which calcium and sodium ions stream into the cell. This flow of positively charged ions temporarily changes the potential difference across the cell membrane: a voltage change that is precisely the sort of electrical signal that ultimately makes its way into the central nervous system.
 There is one idiosyncrasy in the effect of capsaicin on the receptor, though: the phenomenon of desensitization [38], i.e., a decrease in sensitivity that develops with regular occurrence. This explains our observation that people especially from certain cultures, manage to eat extremely spicy dishes that a lot of people are unable to tolerate. Two different effects might be contributing to such habituation.
There is one idiosyncrasy in the effect of capsaicin on the receptor, though: the phenomenon of desensitization [38], i.e., a decrease in sensitivity that develops with regular occurrence. This explains our observation that people especially from certain cultures, manage to eat extremely spicy dishes that a lot of people are unable to tolerate. Two different effects might be contributing to such habituation.
On the one hand, stimulation at regular intervals might lead to some sort of damping of the transmission of the pain signal to the central nervous system [39]. Alternatively, it may be that constant or frequent excitation leads to partial degradation of TRPV1-containing neurons [40].
Overall, the sobering conclusion must be drawn that, as far as the tongue is concerned, hot peppers “taste” exactly like potatoes that are too hot. The perceived pain in both cases causes the brain to warn us: in the one case of an actual high temperature, and in the other of an imaginary one. A glance at the chemical structure of capsaicin (Fig. 12) points us in the direction of a possible treatment for the latter: capsaicin is only slightly polar, so while it should be only slightly soluble in water it could be more soluble in fats. Various antidotes for a burning tongue as a result of spicy food have in fact been compared experimentally [41]. Thus, water – even ice water – turns out to be completely useless, as does beer. Effective agents include cold milk from the refrigerator or ice cream. Presumably, capsaicin is displaced from the receptors by milk proteins, such as casein, and subsequently dissolves in the tiny, oily droplets that are present [42].
<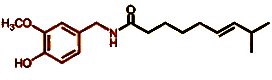
Figure 12. Capsaicin.
5. How Hot is “Hot”?
The relative “hotness” of a pepper or pepper product is an important quality criterion, both for industrial processing and for the individual consumer. A potential buyer will want to know in advance how hot a particular pepper product is, to ensure that its use, presumably in a dish, will give the desired degree of spiciness or “hotness”.
5.1 How Can this Spiciness be Measured?
In 1912, the chemist Wilbur Lincoln Scoville developed a procedure for quantifying levels of hotness while working at the pharmaceutical firm Parke-Davis, Detroit, MI, USA, acquired in 1970 by Warner-Lambert, Philadelphia, USA, which in turn became a part of Pfizer in 2000 [43]:
- 100 mL of pure ethanol is poured over a portion of pulverized pepper fruit (64.8 mg), and the mixture is allowed to stand overnight. After careful shaking, the mixture is then filtered. The filtrate is diluted with sweetened water until it is no longer perceived as hot to the tongue. The corresponding extent of dilution can be regarded as a standardized measure of the fruit’s pepperiness.
Scoville, himself, determined the hotness of peppers from Japan, Kenya, and Zanzibar by using this method, and arrived at  dilution values as high as 1:100,000. In his honor, the unit of measure became known as the Scoville heat unit (SHU). Establishing an SHU value is rather complicated, and somewhat costly as well, because at least six independent testers are required to evaluate the various dilutions. Today, an analytical approach has largely replaced the original organoleptic method. Actual levels of capsaicin and dihydrocapsaicin present are now measured by high-performance liquid chromatography (HPLC) [38, 44].
dilution values as high as 1:100,000. In his honor, the unit of measure became known as the Scoville heat unit (SHU). Establishing an SHU value is rather complicated, and somewhat costly as well, because at least six independent testers are required to evaluate the various dilutions. Today, an analytical approach has largely replaced the original organoleptic method. Actual levels of capsaicin and dihydrocapsaicin present are now measured by high-performance liquid chromatography (HPLC) [38, 44].
A conversion factor was required for transforming measured capsaicin contents into SHUs, so a classical SHU value was determined for pure capsaicin. The Scoville scale is, of course, not “upwardly open”, but must end with the measured hotness level for pure capsaicin, now known to be 16,000,000 SHU (see The Hottest of the Hot).
Pure capsaicin can be safely handled only by adhering to extreme protective measures, e.g., full-body protective clothing that is completely sealed. That still does not prevent the occasional chili fanatic from somehow acquiring an ampoule of pure capsaicin, as the crowning addition to a comprehensive collection of “hot” powders — and even hotter sauces.
5.2 The Hottest Pepper Varieties
 The hottest sort of pepper is currently believed to be Bhut Jolokia, a pepper of the Capsicum chinense variety that is native to the northeast Indian state of Assam and has a SHU value of 1,000,000. Anyone working in the kitchen with these approximately 5 cm long and 1 cm thick pods should always wear gloves, because even the slightest trace introduced into a sore, or onto your eyes, would have extremely painful consequences.
The hottest sort of pepper is currently believed to be Bhut Jolokia, a pepper of the Capsicum chinense variety that is native to the northeast Indian state of Assam and has a SHU value of 1,000,000. Anyone working in the kitchen with these approximately 5 cm long and 1 cm thick pods should always wear gloves, because even the slightest trace introduced into a sore, or onto your eyes, would have extremely painful consequences.
Condiments prepared from peppers are highly prized throughout the world. Tabasco® Sauce, for example, can be used with very little effort to provide just the right degree of spiciness to a dish. Other similar sauces, some rated at more than 1,000,000 SHU, can be extremely “hot”, because if prepared by direct extraction of pepper fruits they can acquire rather high concentrations of capsaicin.
Someone who was already gasping for air after just a few drops of Tabasco® would be regarded among chili fanatics as a sissy, because this sauce — rated at only 3,000 SHU — is actually classed as “rather mild”. For connoisseurs, true delight is only achieved with sauces that high at least five-figure SHU values. Whole books have been written on this subject [45], and the quality of various hot sauces constitutes the basis for endless discussion (and dispute) over the internet [46].
5.3 The Toxicity of Capsaicin
Now we come to a fundamental question among pepper lovers: What is capsaicin’s toxicity?
In 2002, the Scientific Committee on Food of the European Commission actually issued an all-clear signal in this regard [47]. The daily amounts of capsaicin typically consumed in Europe (approximately 1.5 mg), apparently present no danger whatsoever. In countries such as India, Thailand, and Mexico, one could be dealing with daily intakes 100-fold higher, however, and in this case there are indications of increased risk of cancer in the upper digestive tract.
5.4 Vitamin C
Peppers are also a rich source of vitamin C, and can thus be regarded as especially valuable “vegetables”. The Hungarian scientist Albert Szent-Györgyi was the first to isolate large quantities of this vitamin from peppers, thus permitting its structure to be determined, and later facilitating the development of a profitable industrial synthesis.
This work led to Szent-Györgyi being awarded the 1937 Nobel Prize for Physiology and Medicine (although it might be argued that the peppers themselves actually deserved the award for their synthetic achievement!) [48, 49].
6. Non-Culinary Dimensions of Peppers
Apart from direct consumption as “vegetables” or seasonings, pepper products have many other applications.
6.1 E160c
For example, one of the most important food colorants, registered in Europe as “E160c”, is isolated from peppers. Hidden behind this cryptic abbreviation – dismissed by many consumers as merely “chemistry again” … – is nothing more than a specific dye extract derived from paprika, consisting largely of a mixture of the paprika ketones capsanthin (8) and capsorubin (10) (see Fig. 13).
.jpg)
Figure 13. Sources of color in peppers.
The food industry takes advantage of E160c’s bold red color in cereals, beverages, sauces, soups, and confections. It may be that certain critical consumers reject these products for reasons of taste, the cause should certainly not be their E160c content, because this dye is strictly a natural product.
6.2 Pepper Sprays
The pharmacological efficacy of “hot” substances has led to interesting products, as well as to some imaginative and even bizarre applications. For example, a relatively concentrated capsaicin solution, supplied in an aerosol can, constitutes a remarkably effective weapon, one that can temporarily incapacitate an adversary without risk of long-term injury. These so-called pepper sprays (commonly also referred to by the trade name “mace”) fall under the German Weapons Law, and – assuming reasonable use – they can be deployed by the police.
The unpleasant effects of capsaicin are felt not only by humans, but also by many animals. Thus, pepper sprays are sometimes carried by postal delivery personnel as defense against aggressive dogs and the compound is also useful against species such as porcupines and martens, which enjoy chewing on the insulation around electrical wires and cables found in automobiles (here the trick is to rub the threatened wiring with a capsaicin salve).
Wild animals, ranging from deer to elephants, can be discouraged from grazing on ornamental plants and crops by using appropriate forms of this agent [50], and small rodents, such as squirrels, can be similarly put off from stealing bird seed. Birds are not affected by capsaicin, because they lack the appropriate receptors. As a result of its capsaicin content, paprika is only eaten by birds, and not small mammals, which can digest the seeds – or at least alter them such that they no longer germinate.
Birds, on the other hand, excrete most of the seeds unchanged and still capable of germinating in the shade of the donor tree, where the germlings stand a good chance of thriving [51, 52].
6.3 Therapeutic Applications
Capsaicin also finds uses in a therapeutic context. Noteworthy examples include the “Hansaplast® ABC-Heat Plaster”, introduced by Beiersdorf, Hamburg, Germany, in 1928, and the subsequently developed “ABC-Heat Cream” (the term Hansaplast is often used in Europe in a generic sense, in the same way that many in the United States refer generically to a BandAid®) [54]. The heat-plaster material was originally impregnated with Arnica, Belladonna, and Capsaicin (hence, “ABC”), but the formulation was changed a long time ago, such that only the capsaicin remains.
The therapeutic action is associated with its effect on levels of “substance P” (“P” for pain), a very active natural neuropeptide consisting of 11 amino acids. Substance P is a neurotransmitter and neuromodulator that facilitates signal transduction in the central nervous system. Moreover, it leads to vasodilation and thus improved blood flow, which, at least within limits, is perceived as a “warm glow”. Finally, substance P induces formation of endorphins, the body’s own morphine-like compounds, and these act as analgesics (pain relievers).
Application of an “ABC” plaster or cream leads first to a powerful “burning” sensation at the site of the treated skin (nociceptors), followed by a pleasant feeling of warmth (improved blood flow), and finally a pain-relieving effect (endorphins).
6.4 Doping Agent
Capsaicin’s ability to stimulate blood flow can also be taken advantage of in equestrian sports; sometimes the front legs of jumping horses in training are rubbed with a capsaicin-containing salve. The resulting increased blood flow leads initially to sensitization toward pain, such that the horse will find any contact with a hurdle or other obstacle to be extremely painful. During warm-up f or show jumping, the rider might deliberately ride the treated horse up to a hurdle incorrectly. The horse would then inevitably collide with the hurdle very painfully! The result would be that, in a subsequent contest, the poor horse, out of sheer fright, would try to raise its legs especially high, thus clearing all the hurdles easily. For this reason, capsaicin is now considered a forbidden doping agent.
or show jumping, the rider might deliberately ride the treated horse up to a hurdle incorrectly. The horse would then inevitably collide with the hurdle very painfully! The result would be that, in a subsequent contest, the poor horse, out of sheer fright, would try to raise its legs especially high, thus clearing all the hurdles easily. For this reason, capsaicin is now considered a forbidden doping agent.
The 33-year old Christian Ahlmann, a member of the German show-jumping team and European champion for 2003, was disqualified from the 2008 Olympic Games because he apparently applied capsaicin to his 15-year-old gelding Köster. Of course, neither the rider nor handler had any idea how capsaicin could have come to be present on Köster’s legs. In the same Olympic competition, three other riders were also disqualified because of capsaicin misuse. This included the Norwegian Andre Hansen and caused Norway to lose a bronze medal to Switzerland. The fact that capsaicin was shown to be present on four of the 15 horses tested suggests there must have been “a strange epidemic”, because it is surely unlikely that these horses would, for example, have shared a common tube of toothpaste!
Acknowledgments
I am grateful to Dr. S. Streller and Dr. P. Winchester of the Freie Universität Berlin, Germany, for their support and assistance.
References
[31] A. Fein, cell.uchc.edu/pdf/fein/nociceptors_fein_2012.pdf
[32] A. I. Basbaum, D. Julius, Spektr. Wissensch. 2007, July, 44. Link
[33] Michael J. Caterina, Mark A. Schumacher, Makoto Tominaga, Tobias A. Rosen, Jon D. Levine, David Julius, Nature 1997, 389, 816–824. DOI: 10.1038/39807
[34] A. Maelicke, Nachr. Chemie 2000, 48, 946–949. DOI: 10.1002/nadc.20000480717
[35] D. E. Clapham, Nature 2003, 426, 517–524. DOI: 10.1038/nature02196
[36] W. Greffrath, Der Schmerz 2006, 20, 219–225. DOI: 10.1007/s00482-005-0440-9
[37] D. B. Rusterholz, J. Chem. Educ. 2006, 83, 1809–1815. DOI: 10.1021/ed083p1809
[38] A. Szallasi, P. M. Blumberg, Pharmacol. Rev. 1999, 51, 159–212. Link
[39] Vincenzo Di Marzo, Peter M Blumberg, Arpad Szallasi, Curr. Opin. Neurobiol. 2002, 12, 372–379. DOI: 10.1016/S0959-4388(02)00340-9
[40] M. Fitzgerald, Pain 1983, 15, 109–110. DOI: 10.1016/0304-3959(83)90201-4
[41] C. W. Nasrawi, R. M. Pangborn, Physiol. Behav. 1990, 47, 617–623. DOI: 10.1016/0031-9384(90)90067-E
[42] R. Henkin, JAMA, J. Am. Med. Assoc. 1991, 266, 2766. DOI: 10.1001/jama.1991.03470190114047
[43] W. L. Scoville, J. Am. Pharm. Assoc. 1912, 1, 453–454. DOI: 10.1002/jps.3080010520
[44] T. A. Betts, J. Chem. Educ. 1999, 76, 240. DOI: 10.1021/ed076p240
[45] J. T. Thomson, The Great Hot Sauce Book, Ten Speed Press, Berkeley, USA, 1995. ISBN: 9780898157833
[46] www.pepperworld.com
[47] Opinion of the Scientific Committee on Food on Capsaicin, European Commission 2002, Feb 28.
[48] Sabine Streller, Klaus Roth, Chem. Unserer Zeit 2009, 43(1), 38–54. DOI: 10.1002/ciuz.200900481
[49] Klaus Roth, Sabine Streller, Vitamin C Deficiency, ChemViews Mag. 2014. DOI: 10.1002/chemv.201300134
[50] A. M. Rouhi, Chem. Eng. News 1996, 74 (10), 30. DOI: 10.1021/cen-v074n010.p030
[51] J. J. Tewksbury, G. P. Nabhan, Nature 2001, 412, 403–404. DOI: 10.1038/35086653
[52] Annette Hille-Rehfeld, Chem. Unserer Zeit 2008, 42, 306. DOI: 10.1002/ciuz.200890056
[53] www.hansaplast.de
Author
Professor Klaus Roth, Freie Universität Berlin, Germany.
The article has been published in German in:
and was translated by W. E. Russey.
The Biochemistry of Peppers
How is it that only plants from the genus Capsicum are able to synthesize compounds that sting one’s tongue so intensely?
► See all articles by Klaus Roth published by ChemistryViews magazine




