Mankind consumes a great deal of tobacco, most commonly in the form of cigarettes. Worldwide, roughly 15,000 kg of nicotine from tobacco makes its way daily into smokers’ lungs. That alone is sufficient reason for us to consider this particular natural product more closely.
In this part we look at how the tobacco plant actually makes nicotine.
7. How Does a Tobacco Plant Synthesize Nicotine?
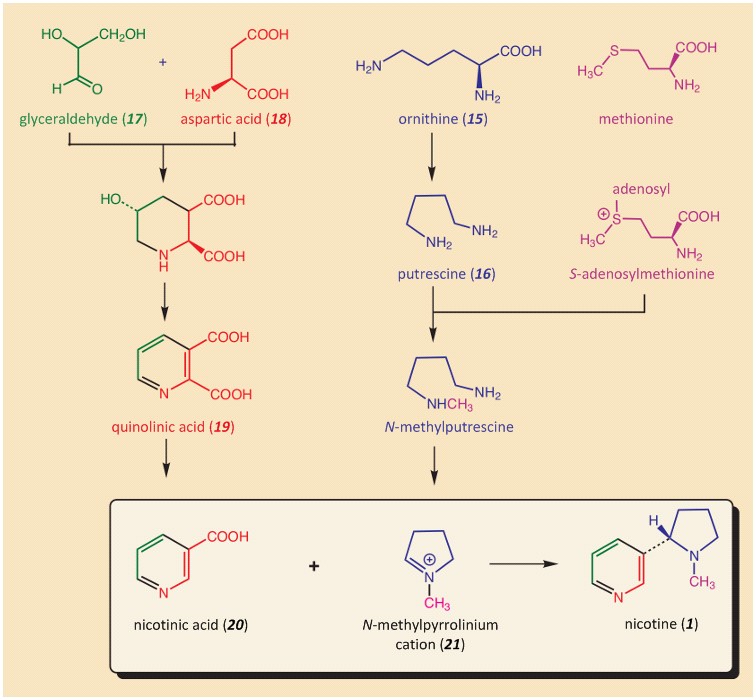
Our knowledge of nicotine biosynthesis is based above all on introducing isotopically labeled compounds into the plant metabolism, and then meticulously tracing the label in the resulting metabolites. The overall picture produced through many, many experimental studies [14,15] shows the masterful way that a tobacco plant, starting from three general-purpose amino acids, (aspartic acid, ornithine, and methionine), along with glyceraldehyde, a degradation product of glucose, constructs the pyridine and pyrrolidine portions separately, finally combining them to give nicotine (see Fig. 3). Nicotine is produced in the roots of the plant and accumulates it in its leaves up to a dry-weight concentration of as much as 9 %!
|
|
|
Figure 3. Biosynthesis of nicotine in a tobacco plant. |
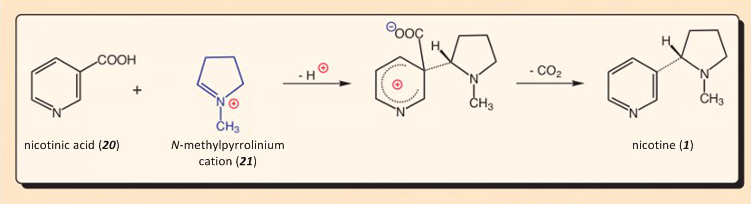
The crowning touch in the biosynthesis, coupling N-methylpyrrolinium cation (21) with nicotinic acid (20) (see Fig 3.), gave chemists in the 1950s a real series of headaches [16]. The reaction mechanism that suggested itself was electrophilic substitution on an aromatic system, but all experience from heterocyclic chemistry argued that such a reaction could not possibly occur at room temperature!
The great British natural product chemist Sir Robert Robinson, who in 1947 was honored with the Nobel Prize in chemistry “for his investigations of biologically important plant products, especially alkaloids”, regarded it as simply impossible for nicotinic acid to be the source of the pyridine ring in nicotine, a position he justified as follows:
- Substitution reactions on pyridine are possible only under extremely drastic reaction conditions.
- Direct replacement with some other substituent of a carboxyl group bonded to pyridine had never before been observed.
Robinson’s voice carried weight not only in those days; his arguments are still impressive today. Thus, bromination of pyridine to give 3-bromopyridine requires a temperature of 300 °C, nitration is possible with potassium nitrate only in fuming sulfuric acid at 370 °C, and Friedel–Crafts reactions with pyridine simply do not occur. Undeterred, the tobacco plant ignores the rule, and proceeds to synthesize large amounts of nicotine at 25–30 °C.
So how does a tobacco plant accomplish this reaction at room temperature?
The answer is simple. Tobacco plants are unable to synthesize nicotine via an electrophilic substitution on nicotinic acid. They only seem to do so. In actual fact, the plant uses the same starting material, and achieves the right result, but in between, a tobacco plant takes a shrewd and intriguing chemical detour. It proved possible to unravel the simply ingenious trick played by the plants only through implementation of a whole series of prudent labeling experiments [9]. Here we briefly analyze the key steps involved:
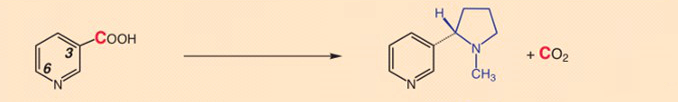
- Experiment 1: The 14C-labeled carboxyl group of nicotinic acid (20) is not incorporated into the nicotine, but eliminated as CO2 [17].
|
|
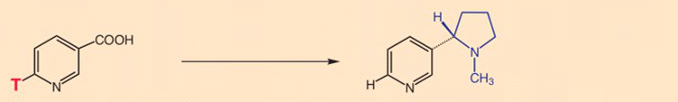
- Experiment 2: Carbon atoms 2 and 3 maintain their positions in nicotine. Nicotine formation thus amounts to a true substitution for the COOH-group.
|
|
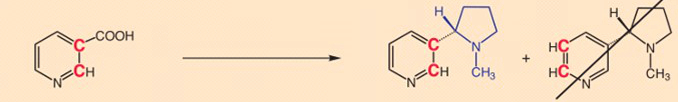
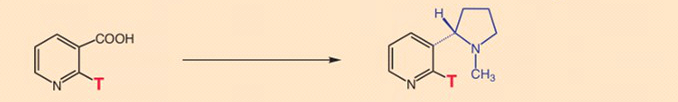
- Experiment 3: Specific tritium labeling at position 2 on the pyridine ring of nicotinic acid shows this atom to be retained in nicotine formation. In other words, during the reaction in question, there is no exhange here of tritium for hydrogen [18].
|
|
- Experiment 4: Now comes the surprise: A tritium incorporated at the 6-position of nicotinic acid is completely replaced during nicotine synthesis by the plant! [19]
|
|
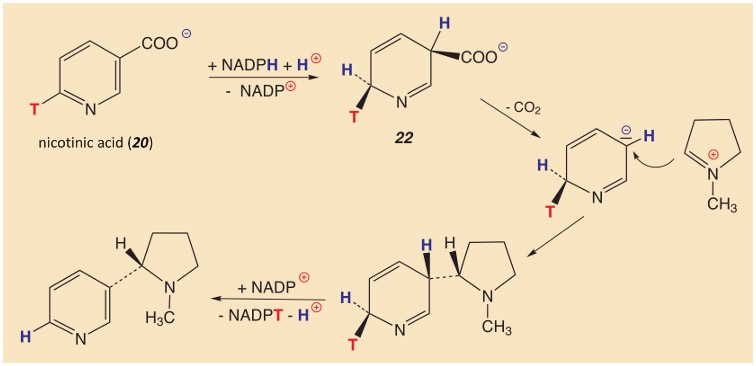
Total loss of tritium labeling at the 6-position in nicotinic acid is explicable only through a temporary loss of aromaticity in the pyridine ring as a result of 3,6-hydrogenation (see Fig. 4) [20].
|
|
|
Figure 4. The amazing heterocyclic chemistry of the tobacco plant. |
The appropriate strong (biochemical) reducing agent is NADPH, flippantly called “the sodium borohydride of biochemistry” [21]. One can so far only speculate about the details of the rest of the reaction process, since the catalytic enzyme in question, nicotine synthase, has to date been only slightly characterized, and isolation has not yet proven possible.
The resulting 3,6-dihydropyridine-3-carboxylic acid (22) is no longer aromatic, and is readily decarboxylated, leading to an azapentadienyl anion (Fig. 5). Cation (21) attacks the latter at the negative C-3, and this leads to a substitution reaction. In the subsequent dehydration, the tritium label is eliminated completely, resulting in a stable pyridine ring [22]. Overall, the oxidizing agent NADP+ arising in the first step is once again consumed. What an elegant and resource-conserving redox cycle!
|
|
|
Figure 5. Lost in reaction. |
8. Why Does the Tobacco Plant Produce Nicotine?
Thanks to this elaborate nicotine synthesis, the plant acquires a significant advantage in the battle to survive, because nicotine is poisonous, and it repels or annihilates predators. Since all plants suffer from a chronic nitrogen deficiency, nicotine production is adjusted upward only upon demand: i.e., when predators nibble on the leaves.
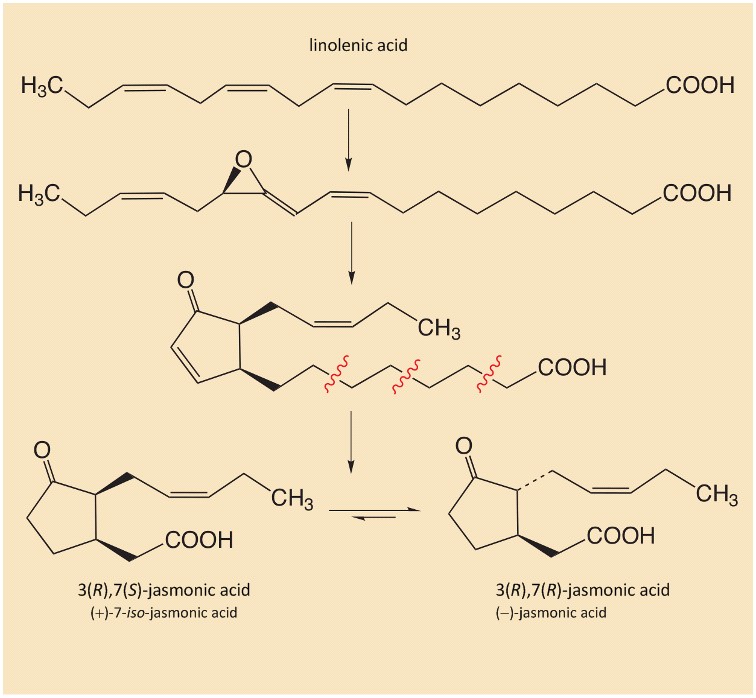
At the site of damage, chemicals from the saliva of the attacking insects (e.g., glutamine) react with fatty acids from the destroyed plasma membranes. The resulting reaction products trigger the defense mechanism, causing formation from linolenic acid of jasmonic acid (see Fig. 6).
|
|
|
Figure 6. Synthesis of the plant hormone jasmonic acid. |
The plant hormone jasmonic acid [23] is distributed throughout the plant, even reaching the roots, where nicotine synthesis is exclusively localized. There the jasmonic acid intervenes in the regulation of gene expression, stimulating synthesis of the enzymes required for nicotine synthesis [24–26]. Nicotine is then transported from the roots to the leaves, where it accumulates. Within a few days, nicotine production is operating at full blast, and the tobacco leaf nicotine content doubles.
This chemically complex defense strategy against predators is costly for the plant, however, because it is forced to commit to this end 6 % of its entire nitrogen supply, a resource which is then lacking, for example, when seed formation is called for.
References
[14] E. Leete in Secondary-Metabolite Biosynthesis and Metabolism (Eds.: R. J. Petroski, S. P. McComrick), Plenum Press, New York, 1992. ISBN: 978-0306443091
[15] P. M. Dewick, Medical Natural Products, John Wiley & Sons, Chichester, 2002. DOI: 10.1002/0470846275.ch6
[16] J. B. Friesen und E. Leete, Tetrahedron Lett. 1990, 31, 6295. DOI: 10.1016/S0040-4039(00)97046-1
[17] T. A. Scott und J. P. Glym, Phytochemistry 1967, 6, 505. DOI: 10.1016/S0031-9422(00)82912-X
[18] R. F. Dawson et al., J. Am. Chem. Soc. 1959, 82, 2628. DOI: 10.1021/ja01495a059
[19] E. Leete und Y. Liu, Phytochemistry 1973, 12, 593. DOI: 10.1016/S0031-9422(00)84449-0
[20] A. Katoh, T. Hashimoto, Front. Biosci. 2004, 9, 1577. DOI: 10.2741/1350
[21] J. Clayden, N. Greeves, S. Warren, P. Wothers, Organic Chemistry, Oxford UP, Oxford, 2001. ISBN: 978-0-19-927029-3
[22] F. R. Stermitz, H. Rapoport, J. Am. Chem. Soc. 1961, 83, 4045. DOI: 10.1021/ja01480a022
[23] C. L. Ballaré, Trends Plant Science 2011, 16, 249. DOI: 10.1016/j.tplants.2010.12.001
[24] A. Steppuhn, I. T. Baldwin et al., PLoS Biol. 2004, 2, 1074. DOI: 10.1371/journal.pbio.0020217
[25] A. Katoh et al., Plant Biotechnol. 2005, 22, 389. DOI: 10.5511/plantbiotechnology.22.389
[26] T. Shoji et al., Plant Cell Physiol. 2008, 49, 1003. DOI: 10.1093/pcp/pcn077
The article has been published in German as:
- Starker Tobak – Unsere Lust und Last mit der Zigarette,
Sabine Streller, Klaus Roth,
Chem. Unserer Zeit 2013, 47, 248–268.
DOI: 10.1002/ciuz.201300636
and was translated by W. E. Russey.
The Chemistry of Tobacco – Part 1
Looking at the history of tobacco consumption – from chewing and snuffing to smoking
The Chemistry of Tobacco – Part 2
What does tobacco contain and which chemical changes happen between the harvest and a finished cigarette?
The Chemistry of Tobacco – Part 3
How does a tobacco plant synthesize nicotine?
The Chemistry of Tobacco – Part 4
What does cigarette smoke contain and what does nicotine do to the smoker?
The Chemistry of Tobacco – Part 5
What happens when a smoker tries to quit?
See all articles by Klaus Roth published by ChemistryViews magazine




the arrow direction in Figure 5 is written in a non conventional way. How the stereochemistry of the intermediate resulting from the reaction of the two sub-units in Figure 5 has been proved?
The pyridine ring has been degraded to carboxylic acid to yield proline of known absolute configuration. See Dagne and Castagnoli, J. Medicin Pharmaceut. Chem. 1972, 15, 356 (Also Klyne and Buckingham, Atlas of Stereochem. Vol 1, p 157.)