Mankind consumes a great deal of tobacco, most commonly in the form of cigarettes. Worldwide, roughly 15,000 kg of nicotine from tobacco makes its way daily into smokers’ lungs. That alone is sufficient reason for us to consider this particular natural product more closely.
In this part we look at the constituents of cigarette smoke and the effects of nicotine on smokers.
9. A Trip to the Center of a Lit Cigarette
Smoking a cigarette produces a chemical extravaganza, one that, in terms of complexity, is hard to beat. By way of an overview, we begin with the reactant, tobacco leaves. These have so far been shown to contain over 3800 identifiable compounds [27]. In the product, cigarette smoke, the corresponding number is actually 4800, including 2800 that are not derived from the tobacco plant itself, so they must have been formed during the smoking process [28]. Dear chemist, what more could your heart desire?
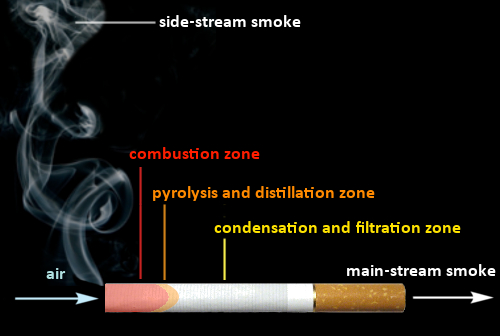
So that we don’t lose track of our chemical overview in the course of smoking an entire cigarette, it is best that we divide it into a series of zones, in which different processes take place (see Fig. 7).
|
|
|
Figure 7. The various zones in a cigarette. |
- In the course of the one or two seconds required to draw in the first 35 mL of fresh air, an increasingly negative pressure develops over the entire span from combustion zone to mouthpiece. The greatest pressure changes occur at the edge of the tobacco, next to the paper wrap, where air velocities up to 400 cm/s are achieved [29] – which after all is equivalent to 12–19 km/h (8–12 mph).
- With the introduction of fresh air, which is rich in oxygen, the temperature rises rapidly in the well-aerated outer layer of the combustion zone, and a bright flaring-up after only 0.1 s makes the highly exothermic oxidation reaction readily visible. In this oxygen-rich zone the tobacco burns completely, leaving behind only ash, with a high mineral content.
- Drawing in more cold outside air leads, after a second or so, to slight cooling of the outer layer of the combustion zone, and as the hot combustion gases are drawn to the center, there occurs a warming of the inner zone, leading overall to a rather complex temperature-distribution pattern [30].
- A temperature maximum is achieved along the central axis, 10–12 mm behind the combustion front. There, and in the adjoining heated pyrolysis zone, an oxygen deficiency prevails, and in this reducing atmosphere the vegetable matter carbonizes. Organic compounds released undergo endothermic dehydration, decarboxylation, dehydrogenation, and cleavage.
- Solid, liquid, and gaseous material formed during carbonization is drawn into the contiguous distillation zone, where it may precipitate as particles or droplets [28].
- Depending on one’s smoking habits, 30–60 seconds may elapse between puffs. During this time, no oxygen is supplied to the tobacco charge, and the combustion zone remains dark, though carbonization processes continue.
10. What Does the Smoker Draw into His or Her Lungs?
Tobacco smoke is an aerosol consisting of gaseous, liquid, and solid materials. It contains between 107 and 1010 solid particles and liquid droplets per mL, where particle diameters are in the range of 0.1–1.0 μm. One is, of course, tempted to inquire what this smoke consists of, since only with such information would it be possible to speculate about why so many people enjoy smoking. Nevertheless, it is apparent that there is no such thing as “the cigarette smoke”. Tobacco types, their processing, and the nature of the paper, additives, and filter materials are simply too diverse.
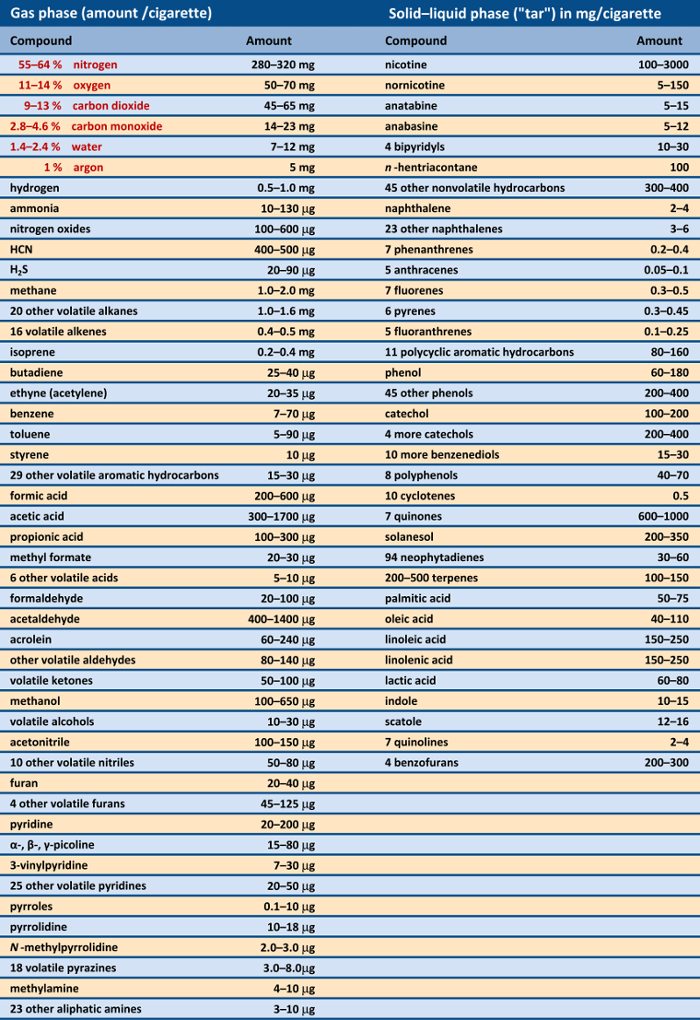
Critical to the content of smoke are the various chemical processes that occur during the act of smoking. A lighted cigarette can be regarded as a complex chemical reactor in which the spatial and temporal distributions of the variables pressure, temperature, and gas velocity, as a function of location, determine the nature of pyrolysis products from the tobacco, additives, and paper. As noted above, about 4800 different chemical compounds have so far been identified in tobacco smoke [31] (see Tab. 1).
|
Table 1. Principal constituents in the inhaled tobacco smoke from a cigarette [31]. |
|
|
Many of the chemical compounds of tobacco smoke are classified as health hazards; indeed, more than 60 are known carcinogens [32]. Countless studies have been published regarding the activity, biodegradation, and associated health risks of individual components of tobacco smoke. Even a superficial treatment of this important but very extensive field would exceed the scope of the present article. For this reason, in what follows we concentrate exclusively on nicotine, since this compound alone is the source of whatever pleasure a smoker experiences, as well as the associated addiction.
11. What Does Nicotine Do to the Smoker?
Of the 10–14 mg of nicotine present in a cigarette, only a small fraction survives the smoking process intact, so that the smoker actually ingests only 1.0–1.5 mg per cigarette. That would seem to be a small amount, but based on estimates drawn from individual casualties, the lethal dose of nicotine for humans is in the range 30–60 mg, making it roughly as toxic as hydrogen cyanide!
Reaching the Nervous System
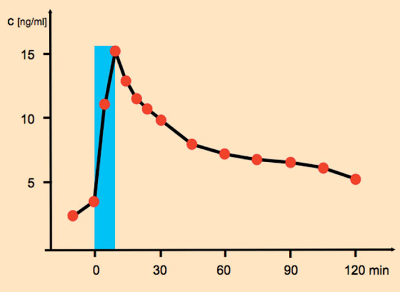
After inhalation of the smoke, nicotine condenses on suspended particles in the fluid of the lungs, which coats the ca. 100 m2 surface area of the 300 million pulmonary alveoli. The nicotine present then penetrates the cell membranes of the alveoli, thereby entering the underlying blood vessels [33]. After passage into the venous portion of pulmonary circulation, the nicotine gains access to the left ventricle of the heart, from which it is pumped throughout the body. Since nicotine in its neutral form passes unhindered through the blood-brain barrier, within 8–10 seconds (!) it reaches the entire central and peripheral nervous system (see Fig. 8) – a rate that would not be surpassed with an intravenous injection! This rapid incorporation enables the smoker to adjust the nicotine level in the blood quite precisely, so as to match rather closely his or her immediate needs: through the frequency, volume, and depth of inhalation on one’s cigarette.
|
|
|
Figure 8. Nicotine concentration in blood plasma after smoking a cigarette (duration of smoking marked in blue). |
Nicotine is above all a powerful neurotoxin, which explains the intensity and extreme breadth of its physiological activity. We limit ourselves here to basic biochemical processes in the nervous system to such an extent that we are able to identify precisely the points of attack of nicotine.
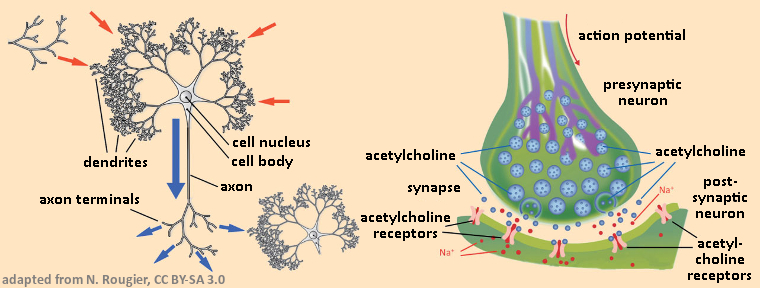
The human nervous system is estimated to contain between 100 billion and 1 trillion neurons (nerve cells). With each of these cells (see Fig. 9), an external stimulus leads to a brief electrical impulse (action potential), which is propagated along a set of fine branchings (dendrites and axons) at velocities between 10 and 300 km/h. A neuron is constructed such that it is directional; i.e., nerve impulses are accepted from the dendrites, processed in the axons, and the result passed from there via the axon terminals to other, neighboring neurons.
|
|
|
Figure 9. Neuronal information processing. |
Signal Transfer at Synapses
The extreme complexity of the nervous system is based above all on the fact that each neuron is able to accept signals from hundreds or thousands of other neurons, and – after processing – may pass the result, also in the form of a signal, to hundreds or thousands of other neurons. Precisely here, at the point of signal transmission from one neuron to another, is where nicotine intervenes. Let us examine how it is that two neurons in fact succeed in communicating with each other across a narrow (20 nm) synaptic cleft. The overall structure involved in the transmission is referred to as a synapse (see Fig. 9, right).
We look first at the events from the perspective of the transmitting neuron. The action potential – a brief variation in potential across the two sides of a membrane – is directly related to the membrane itself, and can thus not be conveniently passed along by way of the synapse, which is filled with water. For this reason, neurons take advantage of an ingenious detour in signal processing.
To begin with, the action potential, as it arrives at an axon terminal, releases a neurotransmitter, which is stored in synaptic vesicles. In the case of the neurons of interest here, this neurotransmitter is acetylcholine, which then diffuses across to the other side of the synapse, where it binds to special acetylcholine receptors in the dendrite membrane of the receiving neuron. A chemical signal generated by this binding changes the spatial structure of the receptor such that an ion channel opens along its principal axis. Through this, sodium ions are able to stream into the intracellular region of the receptor neuron, which results in a change in voltage across the neuron membrane. This is nothing more than a new action potential.
The overall transformation of an electrical into a chemical signal and then back again into an electrical signal would appear to be quite complicated and presumably time-consuming. But not for neurons! The entire process of two-fold transformation from an electrical into a chemical signal (which is to say release of acetylcholine from the transmitting neuron, diffusion of the acetylcholine across the synapse, and creation of an action potential in the receptor neuron) is complete in the course of a few milliseconds.
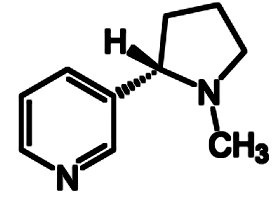
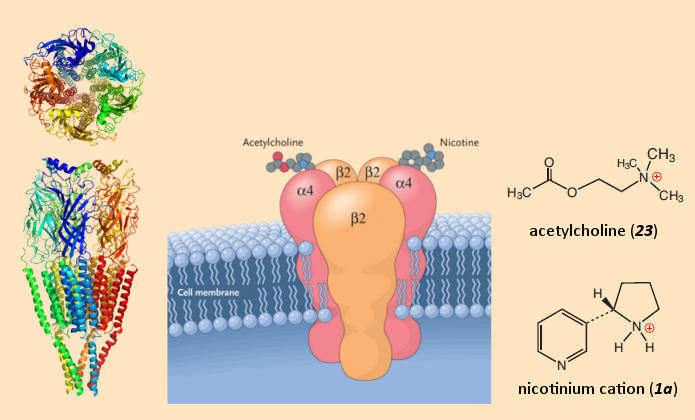
Nicotine interferes severely in signal transfer at the synapse, in that the nicotine itself can bind – instead of acetylcholine – at the acetylcholine receptors. These receptors are known as nicotinic Acetylcholine (nACh)-receptors. The chemical basis for this neurotoxic activity of nicotine is a similarity between the protonated form of nicotine (1a) and the neurotransmitter acetylcholine (see Fig. 10).
|
|
|
Figure 10. The nicotine crime scene: nicotinergic acetylcholine receptors [34,35]. |
But whether it is nicotine or acetylcholine that binds to the nACh makes a great difference: acetylcholine binds to the receptor only very briefly, because within a few milliseconds of its release, extremely active enzymes (esterases) will have broken the acetylcholine down. Nicotine behaves very differently, however, because it is stable, and thus blocks the receptor, which leads to prolonged excitation of the receiving neuron.
The consequences are far-reaching, because nACh receptors are to be found throughout the central and peripheral nervous systems! Many poisoning symptoms – including muscle cramps, nausea, vomiting, diarrhea, and difficulty breathing, all the way to respiratory arrest – can be traced back to disruption of neuronal muscular control caused by nicotine. Elevated heart rate and blood pressure, as well as vasoconstriction and headaches, are further expressions of autonomic nervous system disturbances occasioned by nicotine.
12. How Does Nicotine Make a Smoker Happy?
For a smoker, smoking simply feels good! With the very first puff, nicotine provides a sense of well-being. Depending on the circumstances, the smoker becomes animated or pacified, stays alert or sheds anxieties. Nicotine triggers these positive prevailing moods indirectly through stimulation of the neuronal reward system [36]. Such internal reward systems are evolutionarily quite ancient, and are found not only in humans but also in fruit flies and rodents. They see to such sensations as joy and passion: upon eating when one is hungry, drinking with thirst, or following sex, all of which are crucial activities for the survival of individuals or of the species.
The reward system associated with positive feelings is, in the case of humans, the mesolimbic pathway, a group of mutually cooperative brain structures in the fore- and midbrain. The psychoactive effect of nicotine begins with preferential binding at the nACh receptors of the ventral tegmentum (see Fig. 11), localized in the midbrain.
|
|
|
Figure 11. Nicotine’s attack on the mesolimbic system. |
From here a neuron bundle projects the activities into the Nucleus accumbens, the prefrontal region, and a few other brain structures, which collectively constitute the mesolimbic system. Further transmission of the overall stimulus by way of synapses occurs there through the neurotransmitter dopamine (24). Especially the Nucleus accumbens, regarded as the center of the reward system, releases large amounts of dopamine, accompanied by an immediate sense of well-being.
13. How Does Nicotine Cause the Smoker to Become Addicted?
There is probably no smoker in the world for whom the first cigarette could have been described as “enjoyable”. All first-timers probably found themselves somewhat nauseated and dizzy. Smoke inhalation quite likely caused their lungs to hurt, set them to coughing, and made it seem hard to breathe. Later there may even have been a subsequent bout of diarrhea! All in all it was a rather horrible experience, leading to one universal conclusion: “Never again”. But that was temporary. Why?
There is no way to regard the high percentage of smokers as rational, especially since, in the meantime, everyone knows that smoking is bad for one’s health. Most smokers in fact actually claim they would like to quit: at least someday! Most have probably tried already to quit – many times, perhaps – but without notable success. Prior defeats are repressed, though, and despite knowing better, based on their own experience, many claim, along (allegedly) with Marilyn Monroe: “I can stop anytime I want to, only I don’t want to!”.
 How is it that nicotine causes the smoker to become so addicted? It’s because, with the very first cigarette, nicotine begins to bind to nACh-receptors in one’s nervous system. Apart from the peculiar side effects – nausea, increase in both blood pressure and pulse rate, as well as coughing due to smoke inhalation – the internal reward system begins subliminally to send out a positive signal. The second cigarette already tastes somewhat better, in part because the unpleasant side effects are not as severe, due to familiarization, but also because first withdrawal symptoms are mitigated somewhat by the renewed intake of nicotine. The smoker learns quite quickly from (still subliminal) positive signals from the reward system [36]. Through an emotional learning process, the reward center very quickly recognizes that it “needs” nicotine in order to feel comfortable. Addiction has begun! Inhalation of nicotine results in especially swift addiction above all because an emotional “high” develops within a few seconds after the first drag on a cigarette. Thanks to this essentially instantaneous effect, smoking is the most powerful way there is of becoming addicted to nicotine [6].
How is it that nicotine causes the smoker to become so addicted? It’s because, with the very first cigarette, nicotine begins to bind to nACh-receptors in one’s nervous system. Apart from the peculiar side effects – nausea, increase in both blood pressure and pulse rate, as well as coughing due to smoke inhalation – the internal reward system begins subliminally to send out a positive signal. The second cigarette already tastes somewhat better, in part because the unpleasant side effects are not as severe, due to familiarization, but also because first withdrawal symptoms are mitigated somewhat by the renewed intake of nicotine. The smoker learns quite quickly from (still subliminal) positive signals from the reward system [36]. Through an emotional learning process, the reward center very quickly recognizes that it “needs” nicotine in order to feel comfortable. Addiction has begun! Inhalation of nicotine results in especially swift addiction above all because an emotional “high” develops within a few seconds after the first drag on a cigarette. Thanks to this essentially instantaneous effect, smoking is the most powerful way there is of becoming addicted to nicotine [6].
Chronic smoking also reduces the number of dopamine receptors in the mesolimbic system, such that the stimulus threshold rises for activation of the reward system. This is one reason why cigarette consumption increases as one becomes accustomed to smoking, until the only reason for smoking becomes an escape from withdrawal symptoms. Apart from nicotine, other recreational drugs, including amphetamines, alcohol, cocaine, and opioides like heroin, also stimulate the mesolimbic system, thereby inducing positive sensations. Also relevant here is dopamine release in the Nucleus accumbens; this too results in an attitudinal “high” because of which dopamine is often referred to as a “happiness hormone”. This is misleading, however. It is not simply nicotine (or other drugs) that causes the release of dopamine. Learning processes are the true culprits. A smoker has learned to associate specific situations or activities with a forthcoming “nicotine kick”. The mere sight of a cigarette lighter or an ashtray causes dopamine release to skyrocket. Our reward system reacts in anticipation of a forthcoming “high”, leaving the smoker almost no choice but to fulfill this expectation.
14. What Does a Smoker’s System Do With the Nicotine?
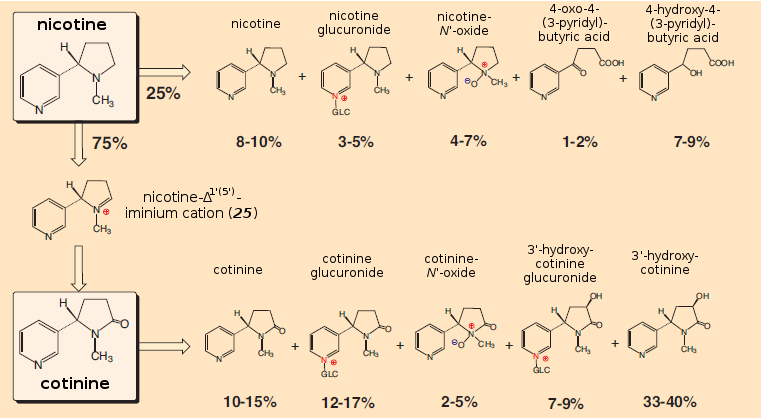
Nicotine is broken down in the body with a half-life of ca. 1–2 hours, although there is considerable variability from one individual to another. In a first step, nicotine is oxidized mainly to iminium cation (25) (see Fig. 12).
|
|
|
Figure 12. Breakdown of nicotine in the smoker’s body. |
This reaction proceeds with help from cytochrome CYP2A6, present in liver microsomes. The rate of decomposition depends, however, on a great many factors, such as gender (women degrade it more rapidly than men), age (the older, the more slowly), various medications, circadian rhythm, pregnancy, the menstrual cycle, etc., etc. Eating and drinking also alter the decomposition rate of nicotine. Thus, increased blood flow in the liver after a meal accelerates decomposition; grapefruit juice, on the other hand, retards the decomposition, because compounds present in grapefruit inhibit cytochrome CPY2A6. It is especially noteworthy in this context that menthol, a common additive in cigarettes, also decreases the rate of nicotine decomposition [37,38].
References
[27] J. C. Leffingwell in Tobacco: Production, Chemistry and Technology, (Eds.: L. D Davis, M. T. Nielsen), Wiley-Blackwell, Oxford, 1999. ISBN: 978-0-632-04791-8
[28] R. R. Baker, Prog. Energy Combust. Sci. 2006, 32, 373. DOI: 10.1016/j.pecs.2006.01.001
[29] R. R. Baker, Nature 1976, 264, 167. DOI: 10.1038/264167b0
[30] R. R. Baker, Nature 1974, 247, 405. DOI: 10.1038/247405a0
[31] D. Hoffmann et al., Chem. Res. Toxicol. 2001, 14, 767. DOI: 10.1021/tx000260u
[32] S. S. Hecht, Nature Rev. Cancer 2003, 3, 733. DOI: 10.1038/nrc1190
[33] C. B. Daniels, S. Orgeig, Physiology 2003, 18, 151. DOI: 10.1152/nips.01438.2003
[34] F. Hucho, Angew. Chem. Int. Ed. 1995, 34, 39. DOI: 10.1002/anie.199500391
[35] F. Hucho und C. Weise, Angew. Chem. Int. Ed. 2001, 40, 3100. DOI: 10.1002/1521-3773(20010903)40:17<3100::AID-ANIE3100>3.0.CO;2-A
[36] N. Birdsall, Drugs and Addiction, Ecstasy and Cannabis.
[37] J.M. MacDougall et al., Chem. Res.Toxicol. 2003, 16, 988. DOI: 10.1021/tx0340551
[38] N.L. Benowitz et al., J. Pharmacol. Exp. Ther. 2004, 310, 1208. DOI: 10.1124/jpet.104.066902
The article has been published in German as:
- Starker Tobak – Unsere Lust und Last mit der Zigarette,
Sabine Streller, Klaus Roth,
Chem. Unserer Zeit 2013, 47, 248–268.
DOI: 10.1002/ciuz.201300636
and was translated by W. E. Russey.
The Chemistry of Tobacco – Part 1
Looking at the history of tobacco consumption – from chewing and snuffing to smoking
The Chemistry of Tobacco – Part 2
What does tobacco contain and which chemical changes happen between the harvest and a finished cigarette?
The Chemistry of Tobacco – Part 3
How does a tobacco plant synthesize nicotine?
The Chemistry of Tobacco – Part 4
What does cigarette smoke contain and what does nicotine do to the smoker?
The Chemistry of Tobacco – Part 5
What happens when a smoker tries to quit?
See all articles by Klaus Roth published by ChemistryViews magazine
.jpg)




In chronic smoking, how is the number of dopamine receptors reduced and how does it infuences activation of the reward system?
This is my fіrst time visіt at here and i am in fact pleassant to read everthing at οne place.