Free-Standing Nanoparticle Monolayers
Free-standing nanoparticle films are of great interest for technical applications, such as the development of nanoelectronic devices. In the journal Angewandte Chemie, Korean scientists have introduced very flexible and stable monolayers of gold nanoparticles made by a self-assembly process based on protein aggregation. The films were used to coat wafers up to 10 cm in diameter.
The success of this new strategy relies on a small protein called α-synuclein, which is responsible for regulation of dopamine release in the brain, among other things. Incorrectly folded forms of this protein, which aggregate into poorly soluble fibril structures, seem to be involved in the development of neurodegenerative diseases such as Parkinson’s. As devastating as this misfolding protein is to the brain, it has shown itself to be quite useful in the production of extensive films made of gold nanoparticles.
Using Proteins to Bind Gold Nanoparticles
To produce these new films, scientists working with Seung R. Paik, Seoul National University, South Korea, first coat gold nanoparticles with α-synuclein. They then adsorb the proteins onto a polycarbonate surface that has been cleaned by treatment with oxygen plasma. The proteins bind to this surface particularly well and eventually build up to form a densely packed monolayer of gold nanoparticles that is held together through unspecific interactions between the proteins. In the final step, the polycarbonate support is dissolved away with chloroform. At the same time, this solvent also triggers the misfolding of the proteins, which allows them to aggregate tightly and specifically, giving the free-standing monolayers necessary stability – even after they are dried. In contrast to previously described methods, this technique can produce films with dimensions reaching the millimeter and centimeter range, such as a 4 inch wafer.
Flexible Films in Different Colors and Shapes
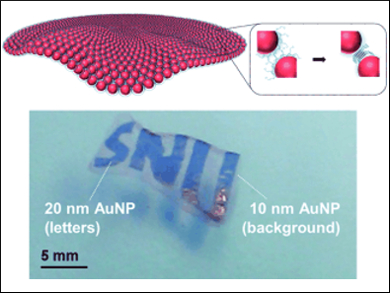
The color of the transparent films depends on the size of the gold particles used: 10 nm particle films are bright pink, 20 nm particle films are purple, and those made from 30 nm particles are dark blue. The films are so flexible that they can be crumpled up and then smoothed out again in a liquid. They can also encase round objects, such as silica spheres, without tearing.
The researchers were additionally able to use lithographically prepared surfaces to make films with patterns of holes. Sequential adsorption on the support also allowed them to make films with a color pattern made from nanoparticles of two different sizes.
The scientists hope to be able to add a variety of functionalities to their films, by using magnetic nanoparticles or quantum dots, for example. Potential areas of application include electronic components, ultrathin displays, and biocompatible sensors for the in vivo observation of organs and tissues. They expect these films to be used for not only controlling cellular activity like cancer treatment, but also cell-to-machine interface in the areas of neuroscience and robotics.
- Free-Standing Gold-Nanoparticle Monolayer Film Fabricated by Protein Self-Assembly of α-Synuclein,
Junghee Lee, Ghibom Bhak, Ji-Hye Lee, Woohyun Park, Minwoo Lee, Daekyun Lee, Noo Li Jeon, Dae H. Jeong, Kookheon Char, Seung R. Paik,
Angew. Chem. Int. Ed. 2015.
DOI: 10.1002/anie.201412461