After having looked at strychnine from historic, medical, and forensic points of view, we sketch how its molecular structure was ascertained.
3. The Tedious Process of Establishing Strychnine’s Structure
The first step taken in the direction of determining the structure of strychnine was establishing its molecular formula: C21H22N2O2, which was accomplished as early as the 1830s [17]. Given that from this molecular formula one could propose millions of distinct isomers, a closer approach to understanding how the 21 carbon atoms were connected required stepwise chemical degradation. Attempts were made to dismantle strychnine using virtually all the reactions available to chemists of the time, in hopes of releasing smaller, simpler molecules with structures that were already known. It was thought that this would at least produce insight into fragments relevant to the overall structural puzzle.
This proved to be an extremely arduous business, particularly because the starting point for each of the degradation reactions was so uncertain. It entailed not only skill, diligence, and stamina, but also a measure of luck, and the latter was apparently not available in large quantities to the chemical “strychninists”, because the journey from molecular formula to structural formula lasted more than a century. Imagine the level of frustration generations of chemists must have experienced when the literally kilograms of crystalline strychnine at their disposal could not be induced to surrender the compound’s secrets [18].
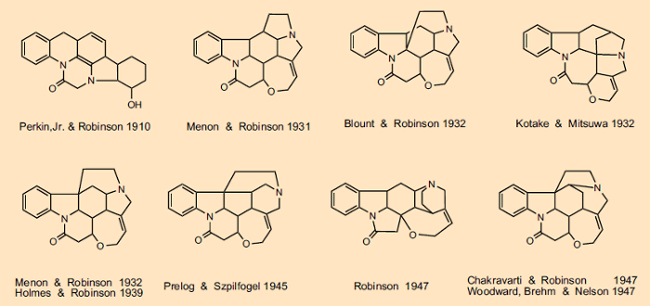
We cannot discuss in detail all the back and forth associated with the many structural suggestions made, but a glance at Figure 2 provides a limited overview of the evolution of strychnine structural formulas. In the course of more than 100 years, thousands of scientists became involved, among them such intellectual giants as Sir Robert Robinson (Nobel Prize 1947), Vladimir Prelog (Nobel Prize 1975), Heinrich Wieland (Nobel Prize 1928), and Robert Burns Woodward (Nobel Prize 1965). Vladimir Prelog, who established in 1945 that the second nitrogen atom was part of a six-membered (not a five-membered) ring, observed in his autobiography that “There is no other organic compound the structure determination of which has exacted so much experimental and intellectual effort as strychnine” [19].
|
|
|
Figure 2. Evolution of the strychnine structural formula. |
With respect to the thrilling competition attending this, the structural Mount Everest of organic natural-product chemistry, there was no shortage among the first-class participants of personal mortification or unceremonious clashes [20]. Thus, Woodward dismissed a structural proposal offered by Robinson in the spring of 1947 as “pure fantasy” [20, 21]. A tit-for-tat response was not long in coming, though. In his December 1947 Nobel Prize lecture, Robinson made explicit mention of Hermann Leuchs, whose group was responsible for 125 publications on the subject of strychnine, and Vladimir Prelog, who contributed the definitive proof that the tertiary nitrogen atom must be part of a 5-membered ring, but not a syllable to Woodward [22], who in the final spurt toward the strychnine structure had made significant contributions, and independently of Robinson had in fact come to the correct result.
4. The First Woodward Total Synthesis – On Paper (1948)
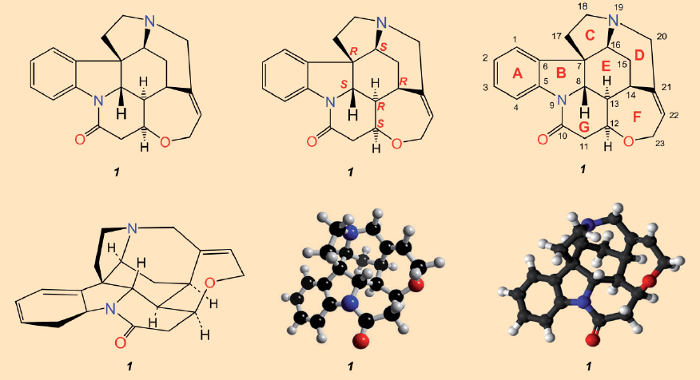
After the isolation of strychnine (1828) and determination of its structure (1947), which was ultimately assured through X-ray analysis in 1950 [23–25], all that was missing as a fitting culmination was a total synthesis. Sir Robert Robinson’s assessment that “for its molecular size, it is the most complex of all substances” [26], implied that accomplishing such a feat had receded far into the distance. A glance at the structural formula in Figure 3 would seem to substantiate his pessimism: the entanglement of seven rings can only be appreciated in a three-dimensional sense after prolonged consideration. With a molecule like strychnine that is so three-dimensionally complex, no single representation is adequate for conveying all the relevant structural details. Synthetic reconstruction of such a labyrinth of carbon atoms looked absolutely impossible.
|
|
|
Figure 3. Chemical structural formulae of strychnine. Top: the most commonly employed structural formula (left), indicating configurations for the six stereogenic carbon atoms (center), and atom numberings along with the alphabetical designations for the seven rings as originally employed by Woodward (right). Bottom: a perspective rendering. |
Only one person dared to tackle this boldest of synthetic challenges: Robert Burns Woodward (1917–1979) [27]. He thought that the most promising approach to developing a synthetic strategy was to follow the lead of nature; that is to say, in everything from starting materials to individual reaction steps, leaning on the synthetic pathway presumably employed by plants. In this case he was treading on rather shaky ground, though, since everyone in those days was quite in the dark about the true biosynthetic origins of alkaloids, given in particular that isotopic methods of study had not yet been developed. The situation changed only in the mid 1950s when techniques were mastered for tracking the fate of individual carbon atoms during metabolism, taking advantage of specific labeling with 13C or 14C atoms in place of the much more common 12C (traces of 14C are detectable by its distinctive radioactivity, and reasonable amounts of 13C by mass spectrometry).
4.1 Biosyntheses of Alkaloids
At the time, generally accepted ideas about alkaloid biosynthesis were based less on experimental evidence than on organic chemists’ imaginations and intuition. Of course not everything was a consequence of grasping at straws: certain laboratory experiences did offer at least some potential for getting one’s bearings. One groundbreaking piece of work in this sense came in 1916 from Amé Pictet and Tsan Quo Chou [28].
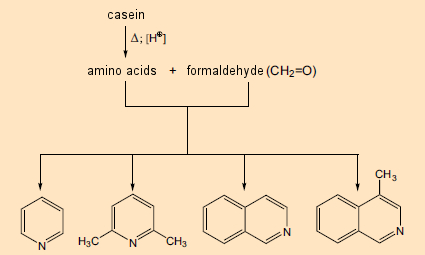
Their study began with casein, a well-known mixture of four proteins, derived from cows’ milk and consisting of 160–210 chemically bonded amino acids. They heated this material for six hours with formaldehyde in aqueous hydrochloric acid. Under such conditions, what occurs first is the release of the individual amino acids, which then react with the formaldehyde, leading among other things to nitrogen-containing heterocycles such as pyridine (see Fig. 4, left) and isoquinoline (right). These were already known to be among the building blocks for numerous plant components, but their own biosynthesis was at the time still a mystery.
|
|
|
Figure 4. Formation of nitrogen-containing heterocycles from proteins and formaldehyde. |
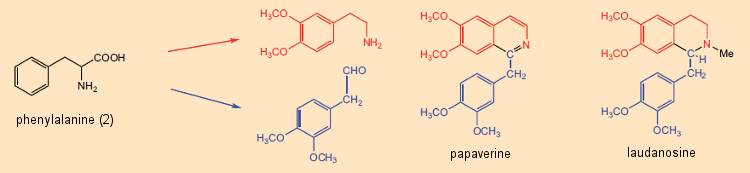
Pyridine and isoquinoline in particular were known to contribute to the structures of a great many alkaloids. As late as the 1960s, this fact served as the basis for a firmly anchored notion that alkaloids were ultimately derived from reaction between free amines (arising from amino acids) and aldehydes, where the aldehydic reaction partner might be a low-molecular-weight metabolic product such as formaldehyde. The synthetic principle “amine (amino acid) + aldehyde (amino acid or metabolite) → alkaloid” was especially attractive due to its simplicity. Indeed, with certain alkaloids such as papaverine and laudanosine, both of which occur in the opium poppy, the role of a pair of amino acid precursors seems quite apparent (see Fig. 5).
|
|
|
Figure 5. The biosynthesis of two alkaloids from the opium poppy: papaverine and laudanosine. |
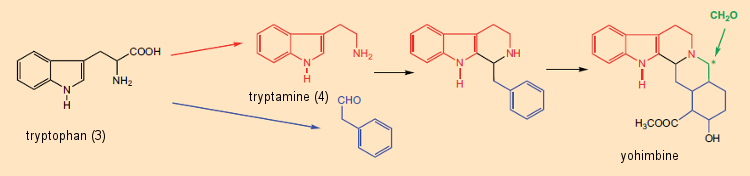
Formal dissection of alkaloids into amino acid components was useful not only in structure elucidation, but also for the reverse: that is, chemists began, on paper, to propose alkaloid syntheses based on this “metabolic principle”. An especially impressive example is the “biosynthetic-like” synthesis suggested by G. Barger and G. Hahn [29,30] of yohimbine (see Fig. 6), found in the bark of the African Pausinystalia yohimbe tree. A plausible biosynthetic pathway to yohimbine could thus be envisioned as involving initial reaction between tryptamine (derived from tryptophan) and phenylacetaldehyde (from phenylalanine), followed by reaction with formaldehyde.
|
|
|
Figure 6. Biosynthetic origin of yohimbine. |
4.2 Woodward’s Initial Plan for a Strychnine Synthesis
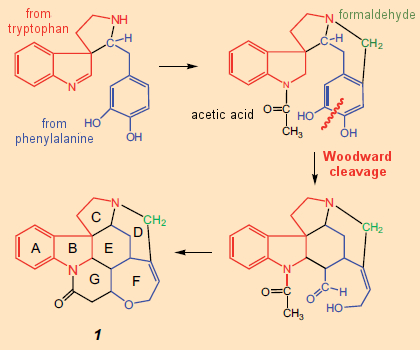
It is only against this background that one can follow Woodward’s initial synthetic plan for strychnine (see Fig. 7). Shaped by the notion that alkaloids are derived in nature from amino acids, elaborated only by relatively small components like formaldehyde or acetic acid, Woodward devised (on paper) the synthetic concept: AB → C → D → E → FG.
|
|
|
Figure 7. Woodward’s initial idea (on paper) for a total synthesis of strychnine (1948). |
Modesty was hardly Woodward’s most conspicuous feature, by the way, as illustrated for example by the ambitious title he assigned to his 1948 publication: “Biogenesis of the Strychnos Alkaloids” [31], whose message he then summarized with the words:
|
“On the whole, the possibility of building up so complicated a structure as (VI) by a series of simple reactions from plausible starting materials is so striking that it is difficult to believe that the scheme lacks significance.” |
In strychnine’s structural formula, a tryptamine subunit (4) would indeed appear unmistakable, constituting rings A and B and a portion of ring C. On the other hand, there are no amino acids immediately obvious within the confusing cluster making up rings D–G. Woodward saw one though: he seized upon an idea proposed in 1948 by Barger and Hahn and was persuaded that he could make out in this tangle a phenylalanine unit, the aromatic ring of which must have been smashed in the course of biosynthesis.
Based on this wild idea as a central feature he published in 1948 – only a year after the structure determination – his thoughts regarding a potential biosynthesis for strychnine (see Fig. 7) [31], clearly noting, however, that this biosynthetic suggestion might not be correct in every detail, and should therefore be interpreted flexibly.
The idea of opening up a phenyl ring was received enthusiastically. For example, in an addendum to Woodward’s publication, Robinson remarked: “The proposed opening of a benzene ring is original in the extreme…. It is apparent that through breaking open a benzene ring and then reassembling the fragments, virtually any structure can be assembled.” Only a few weeks later Robinson himself employed the concept of ring cleavage in the course of solving the structure of emetine, praising Woodward’s “ingenious idea”, and referring to the ring opening step as a “Woodward cleavage” [32].
4.3 Woodward’s Laboratory Total Synthesis (1954)
|
“If we can’t make it, we’ll take it” This comment has been attributed to Woodward, and it certainly is consistent with his sarcastic sense of humor. Whether he actually said it remains uncertain, however. |
By this time Woodward had already synthesized quinine (1944), patulin, and cortisone (1951), and had in 1954 published total syntheses of lysergic acid and lanosterol. But the absolute sensation in this year was his total synthesis of strychnine [33, 34]! Only seven years after elucidation of its structure he and five coworkers succeeded in preparing the natural product – in 29 steps, using known laboratory chemicals. True, the overall yield was less than 0.1 %, but that didn’t matter, since what was important was demonstrating that it was possible in the laboratory to prepare a molecule as complex as strychnine.
For his achievements in the field of natural product synthesis, Woodward was finally rewarded, in 1965, with a long-awaited Nobel Prize in chemistry. The official presentation address, delivered at the award ceremony, concluded with words the level of praise in which could hardly have been exceeded [35]:
|
“It is sometimes said that you have demonstrated that nothing is impossible in organic synthesis. This is perhaps a slight exaggeration. You have, however, in a spectacular way expanded and enlarged the domain of the possible. It is also said that you stand out like a wizard. We know that in times long passed, chemistry was classified as an occult science. Anyhow, you have certainly not gained your scientific reputation by magical means, but by the penetrative intensity of your chemical thinking and the rigorous expert planning of your experiments. In these respects you hold a unique position among organic chemists of today. In recognition of your services to Chemical Science, the Royal Academy has decided to confer upon you the Nobel Prize of this year for your outstanding achievements in the art of organic synthesis.” |
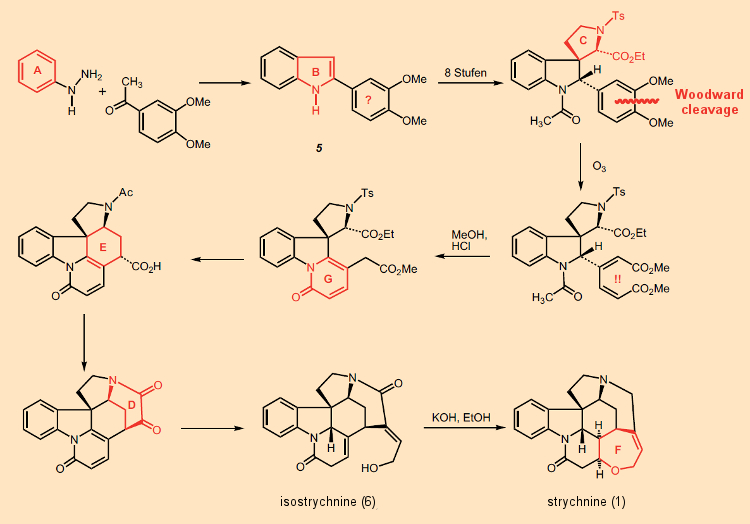
It is not possible for us here to present in detail all the true artistry demonstrated in Woodward’s 1954 strychnine synthesis; but others more qualified have frequently done so already [36, 37]. We limit ourselves instead to the bombshell with which it commences, which stunned chemists then, and does so still (see Fig. 8).
|
|
|
Figure 8. The actual Woodward total synthesis of strychnine (1954). |
To begin with, rings A and B of the indole system were assembled, and then – step by step – rings G, E, and D were added. From the very outset it proved impossible to conform to his original synthetic scheme, namely (AB) → C → D → E → (FG), since a substitution reaction of a phenylalanine unit on tryptophan failed to occur at position 3 as desired, but always at the 2-position instead. So Woodward blocked this position with a phenyl substituent introduced via a Fischer indole synthesis, which made it possible to subsequently construct ring C without interruption; thus, overall: (AB) → C → G → E → D → F.
The last of the rings, F, closes itself in the course of the familiar isomerization of isostrychnine (6) to strychnine [38]. The synthesis starts with a rather furious drumbeat: the seemingly pointless introduction of a dimethoxyphenyl residue on indole 5. The associated “aha moment” comes only after 9 reaction steps, namely with rupture of the phenyl ring using ozone, the two fragments being then utilized as a sort of molecular “quarry” [39] as a way of assembling rings G and E. Simply ingenious!
Introduction of the dimethoxy-substituted phenyl ring at the very beginning using a Fischer indole synthesis does at first appear utterly pointless. Only after several subsequent steps do we see the solution to this confusing puzzle: the aromatic ring is cleaved with ozone. The rather unusual course of action becomes intelligible, however, when viewed against a background of the biochemical synthetic notions posed earlier. That, namely, had led him to regard phenylalanine as a biochemical precursor to strychnine!
4.4 The End of the Woodward Cleavage
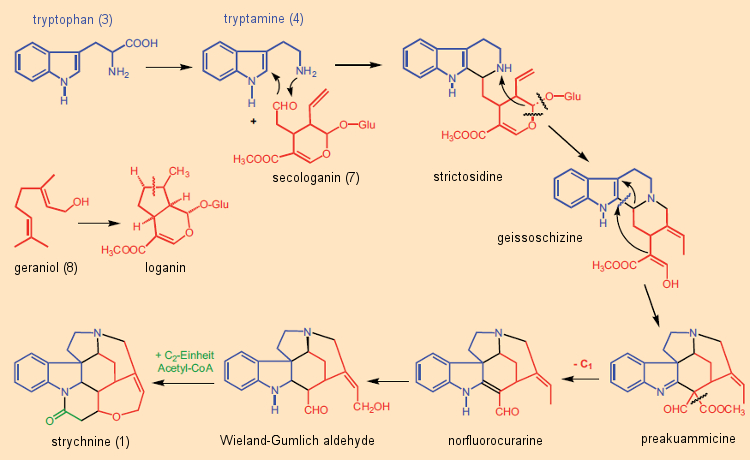
Isotopic studies in the early 1960s showed that in all indole alkaloids the indole portion was derived from the amino acid tryptophan. In most cases this indole unit was constructed from tryptamine – in turn from tryptophan – coupled with the C10 terpene module secologanin.
Many of the more than 3000 examples of such terpenoid indole alkaloids have fascinating complex structures. Secologanin (7) is synthesized in plants from geraniol (8), a key subunit in terpene biosynthesis [40]. This synthetic principle was confirmed through isotopic studies around 1961 by E. Wenkert and R. Thomas [41, 42], representing an abrupt end to the “Woodward Cleavage” idea [43]. Nature indeed synthesizes strychnine in a way different from that originally envisioned by Woodward (Fig. 9): not from tryptophan and phenylalanine, but rather from tryptophan (3) and secologanin (7) is how the “poison nut tree” manufactures its strychnine.
|
|
|
Figure 9. Biogenesis of strychnine in plants. |
Secologanin (7) reacts in the course of a Mannich reaction with tryptamine (4) to give strictosidine, an intermediate in the formation of countless indole alkaloids. The final step in the biosynthesis of strychnine is incorporation of a C2 unit (acetyl-CoA, activated acetic acid) into the so-called Wieland-Gumlich aldehyde (9), followed by a final ring closure.
Summing It All Up
For decades, Robinson, Woodward, and their contemporaries followed the premise that nature makes its alkaloids from amino acids. On this basis, many discoveries could be convincingly explained, important hints were acquired in the course of structure determinations, and synthetic planning was facilitated. Many syntheses planned in this way were implemented successfully, as in the case here of Woodward’s brilliant strychnine synthesis.
From a present-day perspective, the biogenetic pathway Woodward originally postulated for strychnine, centered around cleavage of a benzene ring, was very creative, but it was wrong. Fortunately so, however, since if Woodward had been aware in 1949 of the actual biosynthetic pathway, as shown in Fig. 9, and had he, with the reaction tools then available, attempted a total synthesis based on this knowledge, it is very doubtful that he would have succeeded.
So if we too find ourselves on shaky ground with our thoughts and dreams, let us be both courageous and bold and follow the advice of the great philosopher Karl Valentin: [44]
|
Musical Director: “… incidentally, what do I see there? You don’t have any lenses in your eyeglass frames! … Why do you put on a pair of empty frames? There’s no point in that.” Karl Valentin: “It’s better than nothing at all!” |
References
[17] V. Regnault, Liebigs Ann. Chem. 1838, 26, 10. DOI: 10.1002/jlac.18380260103
[18] S. Berge, D. Sicker, Classics in Spectroscopy, Wiley-VCH, Weinheim, Germany, 2009. ISBN: 978-3-527-32516-0
[19] V. Prelog, My 132 Semesters of Studies in Chemistry, American Chemical Society, Washington D.C., USA, 1991. ISBN: 78-0-8412-1772-0
[20] J. A. Berson, Chemical Discovery and the Logicians’ Program, Wiley-VCH, Weinheim, Germany, 2003. ISBN: 78-3-527-30797-5
[21] R. Robinson, Nature 1947, 159, 263. DOI: 10.1038/159263a0
[22] R. Robinson, Some polycyclic natural products (Nobel Lecture), 1947. http://nobelprize.org/nobel_prizes/chemistry/laureates/1947/robinson-lecture.pdf
[23] J. H. Robertson, C. A. Beevers, Nature 1950, 165, 690. DOI: 10.1038/165690a0
[24] J. H. Robertson, C. A. Beevers, Acta Cryst. 1951, 4, 270. DOI: 10.1107/S0365110X5100088X
[25] A. F. Peerdeman, Acta Cryst. 1956, 9, 824. DOI: 10.1107/S0365110X56002266
[26] R. Robinson, Progr. Org. Chem. 1952, 1, 2.
[27] D. M. S. Wheeler, Chem. Unserer Zeit 1984, 18, 109. DOI: 10.1002/ciuz.19840180402
[28] A. Pictet, T. Q. Chou, Ber. Dtsch. Chem. Ges. 1916, 49, 376. DOI: 10.1002/cber.19160490143
[29] G. Barger, C. Scholz, Helv. Chim. Acta 1933, 16, 1343. DOI: 10.1002/hlca.193301601167
[30] G. Hahn, H. Ludewig, Ber. Dtsch. Chem. Ges. 1934, 67, 2031. DOI: 10.1002/cber.19340671221
[31] R. B. Woodward, Nature 1948, 162, 155. DOI: 10.1038/162155a0
[32] R. Robinson, Nature 1948, 162, 524. DOI: 10.1038/162524a0
[33] R. B. Woodward et al., J. Am. Chem. Soc. 1954, 76, 4749. DOI: 10.1021/ja01647a088
[34] R. B. Woodward et al., Tetrahedron 1963, 19, 247. DOI: 10.1016/S0040-4020(01)98529-1
[35] Professor A. Fredga, Award Ceremony Speech, 1965. http://nobelprize.org/nobel_prizes/chemistry/laureates/1965/press.html
[36] K. C. Nicolaou, E. J. Sorensen, Classics in Total Synthesis, Wiley-VCH, Weinheim, Germany, 1996. ISBN: 978-3-527-29231-8
[37] T. Hudlicky, J. W. Reed, The Way of Synthesis, Wiley-VCH, Weinheim, Germany, 2007. ISBN: 978-3-527-32077-6
[38] V. Prelog et al., Helv. Chim. Acta 1948, 31, 2244. DOI: 10.1002/hlca.19480310750
[39] J. Mulzer, Nachr. Chem. 2007, 55, 731 (in German). DOI: 10.1002/nadc.200746724
[40] P. M. Dewick, Medical Natural Products, 3rd Edition, John Wiley & Sons, Chichester, 2009. ISBN: 978-0-470-74168-9
[41] E. Wenkert, J. Am. Chem. Soc. 1962, 84, 98. DOI: 10.1021/ja00860a023
[42] R. Thomas, Tetrahedron Lett. 1961, 2, 544. DOI: 10.1016/S0040-4039(01)91645-4
[43] A. R. Battersby, Pure Appl. Chem. 1967, 14, 117. DOI: 10.1351/pac196714010117
[44] E. Heilbronner, H. Bock, Das HMO-Modell und seine Anwendung, Verlag Chemie, Weinheim, Germany, 1968. (in German).
The article has been published in German as:
- Die tödliche Brechnuss. Strychnin – von der Isolierung zur Totalsynthese,
Klaus Roth,
Chem. Unserer Zeit 2011, 45, 202–218.
DOI: 10.1002/ciuz.201100552
and was translated by W. E. Russey.
Strychnine: From Isolation to Total Synthesis – Part 1
Just how toxic is strychnine, and why?
Strychnine: From Isolation to Total Synthesis – Part 2
Why did it take 130 years to determine the structure of strychnine?
All arrticles by Klaus Roth published in ChemistryViews
Strychnine: From Isolation to Total Synthesis – Part 3
What can we learn from the total synthesis of strychnine?
Strychnine: From Isolation to Total Synthesis – Interview
Christine Beemelmanns and Hans-Ulrich Reissig explain why the synthesis of strychnine is challenging till today
See all articles by Klaus Roth published in ChemViews Magazine
Also of Interest
- Agatha Christie: The Chemistry of a (Nearly) Perfect Murder,
Klaus Roth,
ChemViews Mag. 2015.
DOI: 10.1002/chemv.201500022
A devilish plan – thwarted by general chemistry knowledge - A Short Route to Strychnine,
Claire D’Andola,
ChemViews Mag. 2015.
Samarium diiodide-induced cascade cyclization - A Modern Synthesis of Strychnine,
Charlotte Brückner,
ChemViews Mag. 2012.
Robert Woodward was the first to make it in 1954 – and you?