In 2010, Christine Beemelmanns and Hans-Ulrich Reissig, Freie Universität Berlin, Germany, succeeded in completing a total synthesis of strychnine. That actually constituted a more thrilling event than may be obvious at first glance. Klaus Roth talked to the researchers about their synthesis in 2010 (see the interview below). We also asked Professor Reissig to give a short summary of what has happened since then.
Interview from 2010
Professor Reissig, could you provide our readers with a brief summary of your field of interest, out of which this strychnine synthesis evolved?
H.-U. Reissig: The goal of our research is developing new synthetic methods for the preparation of bioactive compounds. Heterocyclic compounds are of particular interest. We regard the indole system in particular as being among the so-called “privileged structures”. This ring system appears in an especially large number of active substances, from the neurotransmitter serotonin to the hallucinogen psilocin, from the vasodilator yohimbine to the antihypertensive agent reserpine, and from the cytostatic agent vincristine to the convulsant strychnine: all of these are indoles, along with over 3000 more active compounds.
Dr. Beemelmanns, the title of your dissertation, “Samarium Diiodide-induced Cyclizations of Indole-1-ylketones for Construction of Functionalized N-Heterocycles – A Formal Total Synthesis of Strychnine” [47], would seem to be unusually comprehensive. Could you perhaps help us – briefly – to acquire a better grasp of your topic?
C. Beemelmanns: As Professor Reissig indicated, we have been concerned with developing methods for the synthesis of complex heterocycles. In the first part of my dissertation, I deal systematically with the development and optimization of cyclization methods utilizing samarium diiodide [48]. In this way I was able to attach another lactam ring to a substituted indole, very simply and in high yield. At that point, one of course wants to explore the possibilities opened up by the new methodology, for example, by synthesizing some interesting natural product. With luck, this application is a success. And in the end, I had a great deal of luck.
Professor Reissig, when did you come up with the idea of a new strychnine synthesis?
H.-U. Reissig: I? I didn’t come up with that idea at all! It was Dr. Steffen Gross (Ph.D. in 2003) who approached me with the idea of attempting a strychnine synthesis. Toward the end of his graduate studies, he pursued a first synthetic idea in the direction of strychnine, but unfortunately it did not succeed. We had to abandon this project because he needed to finish up his thesis.
Why in the world would one set off on the thorny path to synthesize strychnine for the nth time? After all, it’s readily available from seeds of the poison nut tree!
H.-U. Reissig: Well, why would one look for an nth new pathway to the top of Mont Blanc? Why does an artist paint a sunflower? Van Gogh did that to perfection a long time ago. Why go to performances of “Tristan and Isolde” with different conductors and orchestras? In organic chemistry, strychnine is something very special, a sort of magic molecule. Just think about how unbelievably many research groups have worked on strychnine, first on its isolation, then with structure elucidation, and finally on total synthesis.
Making one’s own significant contribution is certainly an appealing idea. But it’s not simply a matter of ambition in a sporting sense. One certainly wants to reach the goal, but what really matters is how one gets there. Here, the pathway is the goal, and that entails coming to grips with this highly complex molecule in a simple, elegant and productive way.
C. Beemelmanns: In the case of strychnine synthesis, previously published total syntheses from famous research groups constitute a yardstick, a yardstick one would like to improve on. Having successfully developed a new key synthetic step, I just had the feeling I could do that too. Above all, maybe I could even do it better! That was my main driving force.
When, in your opinion, is one synthesis better than others?
H.-U. Reissig: The evaluation of syntheses is difficult, and always subjective. In general, though, one synthesis is considered “better” to the extent that it is shorter, gives a higher yield, and is “more attractive”.
Shorter and higher yield are easily understood and measurable, but when, from your perspective, is a synthesis “more attractive”?
H.-U. Reissig: An attractive synthesis is one that leads to great complexity in only a few steps. That is the amusing part of our synthesis. Take a look at the key reaction. The starting material really looks quite primitive. No stereocenters, very simple functional groups, but then – all of a sudden – a double ring-closure, and in 77 % yield. What more could one ask for?
C. Beemelmanns: Exactly! In our key reaction step, three adjacent stereocenters are generated, and in the desired configuration. All at once! And the substituent –CH2–CN provides us with all the atoms required for the upper 5-membered ring. These successive ring closures after addition of samarium diiodide (in a “cascade reaction”) are the highlight of the synthesis. Elegant and beautiful!
Dr. Beemelmanns, in the further course of the synthesis you were able to prepare the intermediate already made by Bodwell [49]. So wasn’t your formal total synthesis already complete at that point?
C. Beemelmanns: Right! I was able to demonstrate complete conformity of all the spectroscopic data for my ester with Bodwell’s. That was at the beginning of February, 2010, and I went to the head of the group and said: “We’ve got it!”
Dr. Beemelmanns, that was unfortunately not yet the happy end, because then things became really dramatic.
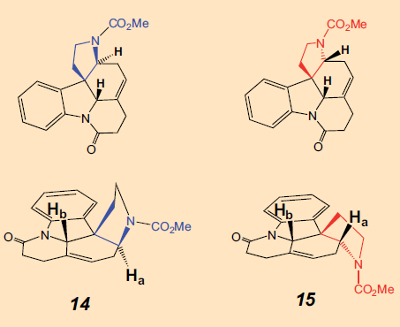
C. Beemelmanns: You can say that again! My dissertation was essentially complete, and we had already celebrated. But because certain signals in the NMR spectra of the ester overlapped, I couldn’t prove with absolute certainty that the upper 5-membered ring was really attached cis (14), and not trans (15). It was necessary that it be cis, because that corresponded to the attachment of the appropriate ring in strychnine. Bodwell had made this assignment for the structure, but I wanted to be certain.
|
H.-U. Reissig: I had already done considerable battle with overlapping signals in my own dissertation with Huisgen, and what often helped me tremendously in those days was switching the solvent from chloroform to benzene, because that caused the NMR signals to shift a little, and with a bit of luck the signals would then separate.
C. Beemelmanns: That did help a little, but only with a change in probe temperature was there a clean signal separation. With the very first spectra, there already was an indication that the two hydrogen atoms Ha and Hb were on the same side of the six-membered ring. (The spatial proximity of the two hydrogen atoms can be verified by the presence of a strong NOE, the nuclear Overhauser effect).
So unfortunately, we had prepared the wrong isomer, 15, and from that, one can’t make strychnine. But Bodwell had the wrong isomer in hand as well. We still had our doubts, though. Perhaps Bodwell had in fact been right, so we looked at the NMR spectra hundreds of times. We went over them yet again, showed them to colleagues, and recorded them once more. Then we took them at different temperatures. I had even begun to dream about NMR signals. But after a month we were sure that neither we nor Bodwell had made 14, but rather the incorrect trans-isomer, 15. My beautiful synthesis was gone. That was really depressing.
So Bodwell’s synthesis had also failed.
C. Beemelmanns: Yes; awful, because proving that was of course never my goal. It is always terribly unpleasant to have to correct a colleague. I would have preferred a thousand-fold that both of us had come up with the right isomer: 14.
And now?
H.-U. Reissig: She was at that point very depressed. And no wonder, because she had nearly reached her goal, only to have this happen. But dead ends are simply a part of scientific research; for her it was an especially bitter lesson. So we allowed ourselves two more months, since Miss Beemelmanns was anxious to finish her dissertation. We soon came up with a “Plan B”, though we were in no way certain that it could in fact be realized, especially within such a limited time frame . But with diligence, skill, and luck Miss Beemelmanns managed very quickly to come up with a five-membered ring with the proper connection, and the overall synthesis was just as short as it had been before. All’s well that ends well!
Have you spoken with Bodwell regarding the assignment of the key intermediate?
H.-U. Reissig: Of course. That’s simply a matter of courtesy. We sent Graham J. Bodwell (Memorial University, St. John’s, Canada) a copy of the manuscript before submitting it, and asked for his reaction. Since I knew him personally it was easier to discuss the matter openly; one shouldn’t forget that we were indirectly asserting that his published “strychnine total synthesis” in truth was none. I wrote to him that I was very sorry this had happened, and that I hoped we wouldn’t have a falling out over it. But we had to publicly correct the error so that our own synthesis would be recognized as successful.
And how did he react?
C. Beemelmanns: He was of course most disconcerted, but reacted quite masterfully. He accepted the “extremely embarrassing” error, and wanted to reconstruct once more how it was that they could have interpreted their NMR spectrum incorrectly.
I found it particularly admirable, by the way, how Bodwell reacted after our paper appeared in Angewandte Chemie [54]. He congratulated us on a “beautiful piece of chemistry”, and thanked Professor Reissig for his extremely professional and considerate handling of this scientific discrepancy, acknowledged the correction of his structural assignment, and saw in the situation a perfect example of self-correction within our discipline. This reaction was simply exemplary.
Professor Reissig, you dedicated the synthesis to your graduate advisor, Professor Rolf Huisgen, in honor of his 90th birthday. Was he pleased by this dedication of a synthesis of one of the most toxic of all natural products to him? That might also be easily misunderstood.
H.-U. Reissig: He was delighted by it, and described our double ring-closure as elegant and a “real blockbuster” in his thank-you note. You asked earlier what it was that made our synthesis “beautiful”. Rolf Huisgen said in his letter “We refer to the elegance of a synthesis, and mean by that its ‘unanticipated simplicity'”. Huisgen knows exactly what he’s talking about in this context, because he is all too well acquainted with strychnine: he did his graduate work under Heinrich Wieland on strychnos alkaloids, especially on vomicine, a close relative of strychnine.
He very soon left this field of natural products [55], however, he made many outstanding contributions through his discovery and many further developments of 1,3-dipolar cycloadditions, as well as a host of other truly remarkable studies. In recognition of these accomplishments, he was presented with an honorary doctorate from the Free University of Berlin, Germany, in the context of a “Festkolloquium” in February 2010. Miss Beemelmanns and I thought it entirely appropriate to dedicate our new, elegant strychnine synthesis to such an outstanding chemist on the occasion of his 90th birthday.
Dr. Beemelmanns, Professor Reissig, I wish to thank you most heartily for this open, collegial conversation.
What has happend since 2010
Professor Reissig, what has happened since then? You recently published an article on a shorter route to strychnine. Can you please say something about your recent research in this field?
Shortly after our communication in Angewandte Chemie, in 2011, a report of Christopher Vanderwal was published, describing a total synthesis of strychnine in only 6 steps (longest linear sequence) [50]. Despite the astonishing conciseness of this route, one of its drawbacks is the caprice of one of the key steps. An organocatalytic approach to strychnos alkaloids including strychnine by David MacMillan et al. was also reported in 2011 [52].
All these new syntheses were apparently a motivation for Larry Overman and his co-worker to publish a review on this topic with the meaningful title “Why is there no end to the total syntheses of strychnine? Lessons to be learned for strategy and tactics in total synthesis”. [56] The authors nicely reflect why molecules such as strychnine serve as benchmarks in organic synthesis. Another successful approach to strychnine was reported just recently, underlining the validity of Overman´s statement [53].
We were also keen to improve and streamline our strychnine approach. Our initial route required 10 steps in the longest linear sequence. By improving the elimination step involved, we now have an overall yield of 14 %, which is still one of the best! However, we also examined a shorter pathway by avoiding a protective group and hence successfully shortened our synthesis to 8 steps, now with an overall yield of 10 %. All of these results were recently published [51]. We also summarized our strychnine story in a personal account, describing our trials and errors and the final success [57].
Professor Reissig, thank you for the update.
References
[47] C. Beemelmanns, Ph.D. Thesis 2010, Freie Universität Berlin, Germany.
[48] C. Beemelmanns, H.-U. Reissig, Pure Appl. Chem. 2011, 83, 507. DOI: 10.1351/pac-con-10-09-06
[49] G. J. Bodwell, J. Li, Angew. Chem. Int. Ed. 2002, 17, 3261.
DOI: 10.1002/1521-3773(20020902)41:17<3261::AID-ANIE3261>3.0.CO;2-K
[50] D. B. C. Martin, C. D. Vanderwal, Chem Sci. 2011, 2, 649. DOI: 10.1039/c1sc00009h
[51] C. Beemelmanns, H.-U. Reissig, Chem. Eur. J. 2015, 21, 8416. DOI: 10.1002/chem.201500094
[52] D. W. C. MacMillan et al., Nature 2011, 475, 183–188. DOI: 10.1038/nature10232
[53] S. Canesi et al., Chem. Eur. J. 2015, 21, 7713–7715. DOI: 10.1002/chem.201500185
[54] C. Beemelmanns and H.-U. Reissig, Angew. Chem. Int. Ed. 2010, 49, 8021. DOI: 10.1002/anie.201003320
[55] R. Huisgen, The Adventure Playground of Mechanisms and Novel Reactions, American Chemical Society, Washington D.C., USA, 1994. ISBN: 0-8412-1832-3
[56] J. S. Cannon, L. E. Overman, Angew. Chem. Int. Ed. 2012, 51, 4288–4311. DOI: 10.1002/anie.201107385
[57] H.-U. Reissig, C. Beemelmanns, Chem. Rec. 2015. DOI: 10.1002/tcr.201500024
The article has been published in German as:
- Die tödliche Brechnuss. Strychnin – von der Isolierung zur Totalsynthese,
Klaus Roth,
Chem. Unserer Zeit 2011, 45, 202–218.
DOI: 10.1002/ciuz.201100552
and was translated by W. E. Russey.
Strychnine: From Isolation to Total Synthesis – Part 1
Just how toxic is strychnine, and why?
Strychnine: From Isolation to Total Synthesis – Part 2
Why did it take 130 years to determine the structure of strychnine?
Strychnine: From Isolation to Total Synthesis – Part 3
What can we learn from the total synthesis of strychnine?
Strychnine: From Isolation to Total Synthesis – Interview
Christine Beemelmanns and Hans-Ulrich Reissig explain why they developed the 17th total synthesis of strychnine
See all articles by Klaus Roth published in ChemistryViews Magazine
Also of Interest
- Agatha Christie: The Chemistry of a (Nearly) Perfect Murder,
Klaus Roth,
ChemViews Mag. 2015.
DOI: 10.1002/chemv.201500022
A devilish plan – thwarted by general chemistry knowledge - A Short Route to Strychnine,
Claire D’Andola,
ChemViews Mag. 2015.
Samarium diiodide-induced cascade cyclization - Clever Picture: Natural Poisons,
ChemViews Mag. 2015.
DOI: 10.1002/chemv.201500039 - A Modern Synthesis of Strychnine,
Charlotte Brückner,
ChemViews Mag. 2012.
Robert Woodward was the first to make it in 1954 – and you?