New Ink Against Counterfeiters
Banknotes, documents, branded products, and sensitive goods like pharmaceuticals or technical components are often marked to distinguish them from imitations. However, some counterfeiters have learned to copy conventional fluorescent tags. In the journal Angewandte Chemie, Chinese scientists have introduced a new anti-counterfeit ink made with carbon nanodots. Their ingenious composite material emits three different types of luminescence.
The team led by Hengwei Lin at the Ningbo Institute of Materials Technology & Engineering of Chinese Academy of Sciences, the University of Chongqing, and Southeast University in Nanjing, has successfully produced such a substance based on carbon nanodots – luminescent nanomaterials which have attracted much attention in recent years due to their unique optical properties and extremely low toxicity.
Carbon Nanodots with a Triple Glow
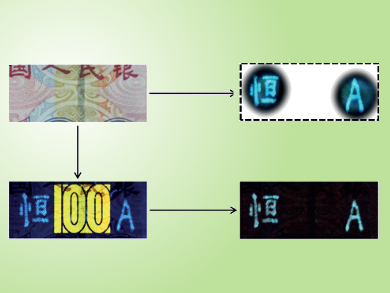
The researchers used a facile process to make carbon nanodots from m-phenylenediamine. These were then dispersed in water with polyvinyl alcohol and dispensed as ink from a gel pen onto a banknote and a document. After drying, the result was a transparent film of carbon nanodots in a polyvinyl alcohol matrix.
This film is colorless under ordinary light, but has three tricks up its sleeve: 1) Irradiation with a UV lamp (365 nm) causes the mark to emit blue light (photoluminescence); 2) the UV irradiation also results in a green afterglow that continues for several seconds after the UV lamp is switched off (room temperature phosphorescence); and 3) irradiation with an infrared femtosecond pulse laser (800 nm) induces a blue-green glow (two-photon luminescence).
Luminescence and Phosphorescence
Photoluminescence is a phenomenon that is widely observed. Irradiation with UV light catapults electrons into a higher energy level. As the electrons return to the ground state, a portion of the energy is re-emitted as visible light. Two-photon luminescence is a significantly less common phenomenon in which two electrons are absorbed simultaneously (in this case in the infrared range) and jumps to a higher level. From this higher level, the electron can return directly to the ground state by emitting light of a shorter wavelength (in the visible range).
Phosphorescence at room temperature is especially rare. It involves a delay in the release of the absorbed energy because quantum mechanically “forbidden” – and therefore unlikely – electronic transitions are involved. The scientists determined that nitrogen-containing groups on the surface of the carbon nanodots are critical to this observed phosphorescence. The embedding of the nanodots in the polyvinyl alcohol matrix is also important, because it inhibits intramolecular motion that works against the phosphorescence.
- Triple-Mode Emission of Carbon Dots: Applications for Advanced Anti-Counterfeiting,
Kai Jiang, Ling Zhang, Junfeng Lu, Chunxiang Xu, Congzhong Cai, Hengwei Lin,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201602445