Vasily V. Krivyk, Nikolai A. Ustynyuk, and colleagues, A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, Moscow, have determined the structures of the intermediates and final products of the reaction of vinylidene complexes of manganese (Ср(CO)2Mn = C=C(H)Ph) and rhenium (Cp(CO)2Re = C=C(H)Ph) with trialkyl phosphites, phosphonites, and phosphinites (P(OR)R’R” (OR = R’ = R” = OMe, OEt, OPri, OPh; OR = OEt, R’ = R” = Ph; OR = R’ = OEt, R” = Ph)).
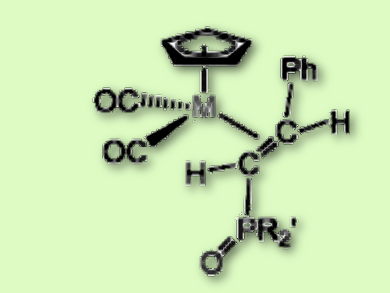
The team found that the reaction does not proceed according to the Michaelis-Arbuzov reaction mechanism, as previously thought. Instead, during the synthesis of the styrylphosphonate complexes by-products form. P–OR nucleophiles add to the Cα atom of Mn and Re vinylidenes to afford the zwitterionic adducts Cp(CO)M¯–C(+P(OR)R′2) = C(H)Ph. Their decomposition in traces of water leads to the formation of the desired phosphorylalkenes Cp(CO)2Re{η2-E-HC[P(O)R′2] = C(H)Ph} (pictured). Isotopic label experiments with D2O and H218O showed the hydrolysis proceeds with the phosphorus–oxygen bond cleavage and binding of the water hydrogen to the carbon atom bearing the >P=O group.
According to the researchers, their findings may be used to develop methods to prepare vinylphosphonate derivatives from terminal alkynes, which is important for organic synthesis.
- Adducts of Mn and Re vinylidenes with P–OR nucleophiles: Hydrolysis rather than the intramolecular Michaelis–Arbuzov rearrangement,
Kamil I. Utegenov, Vasily V. Krivykh, Oleg S. Chudin, Alexander F. Smol’yakov, Fedor M. Dolgushin,Oleg V. Semeikin, Nikolai A. Shteltser, Nikolai A. Ustynyuk,
J. Organomet. Chem. 2017.
http://dx.doi.org/10.1016/j.jorganchem.2017.10.008