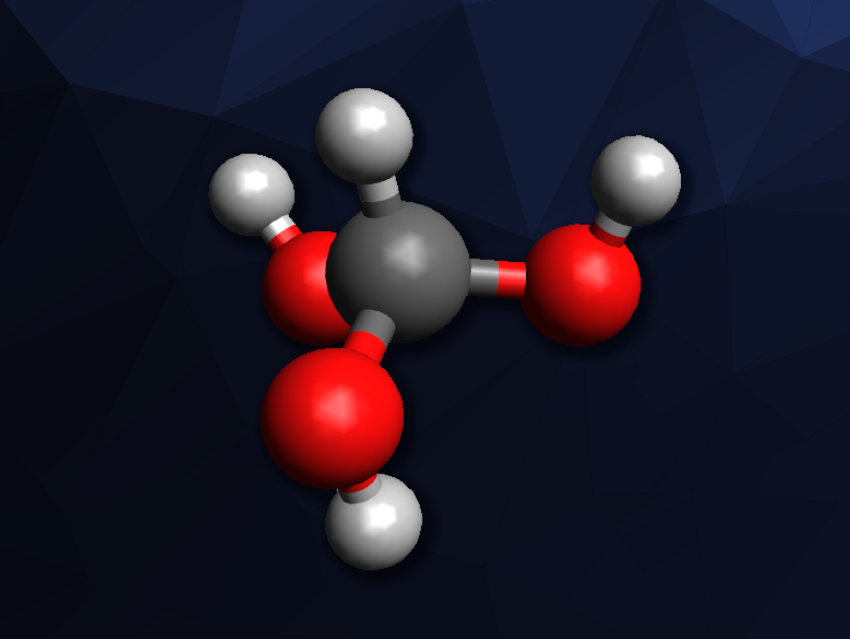
Organic compounds with more than one hydroxyl group bound to the same carbon atom are generally not stable and have a tendency to undergo dehydration reactions. Thus, geminal diols, orthocarboxylic acids, or methanetetrol, which have two, three, or four hydroxyl groups at the same carbon, respectively, can be challenging synthetic targets. Of the simplest compounds of this type—i.e., methanediol, methanetriol, and methanetetrol—only methanediol had been detected in the gas phase so far. Methanetriol and its isomers can play a role in combustion, atmospheric chemistry, and astrochemistry.
Tao Yang, East China Normal University, Shanghai, and Shanxi University, China, Alexander M. Mebel, Florida International University, Miami, USA, Ralf I. Kaiser, University of Hawaii at Manoa, Honolulu, USA, and colleagues have achieved the first synthesis of methanetriol in low-temperature mixed ices of methanol and molecular oxygen subjected to energetic irradiation. The team exposed CH3OH/O2 ices at 5 K to energetic electrons, aiming to mimic energetic galactic cosmic rays. The resulting products were identified after sublimation using vacuum ultraviolet (VUV) photoionization coupled with reflectron time-of-flight mass spectrometry (Re-ToF-MS) with the help of computational predictions of dissociative photoionization pathways.
The team found evidence for the formation of methanetriol as well as its isomers hydroxyperoxymethane and hydroxyperoxymethanol. According to the researchers, the detection of methanetriol as the simplest orthocarbonic acid (RC(OH)3) could allow studies of its chemistry and that of its derivatives. It might also have implications for research in atmospheric chemistry.
- Methanetriol─Formation of an Impossible Molecule,
Joshua H. Marks, Xilin Bai, Anatoliy A. Nikolayev, Qi’ang Gong, Cheng Zhu, N. Fabian Kleimeier, Andrew M. Turner, Santosh K. Singh, Jia Wang, Jiuzhong Yang, Yang Pan, Tao Yang, Alexander M. Mebel, Ralf I. Kaiser,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c02637




Great work.