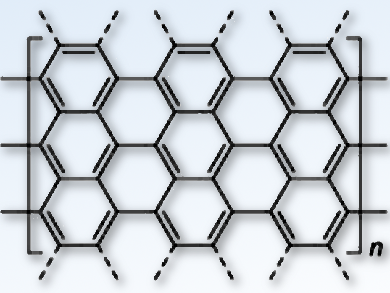
Armchair graphene nanoribbons (aGNRs; pictured) show great promise as high-performance semiconductor materials for use in the next generation of electronic devices. There are many forms of graphene nanoribbon (GNR), but the armchair conformation is one the most studied because of their zero-band gap and high charge carrier mobility. Despite this, a synthetic method that could tailor the width and edge structures for modulating the band gap of these nanoribbons has been lacking.
Guangbin Dong, University of Chicago, IL, and University of Texas at Austin, both USA, X.-Y. Zhu, Columbia University, New York, USA, and colleagues have devised a simple modular method for producing aGNRs with unsymmetrical functionalized edges. A synthetic approach composed of a Suzuki-coupling reaction and cyclodehydrogenation steps created aGNRs with heteroarene (benzothiadiazole and benzotriazole) groups at the edges, so that the band gap could be engineered through an unsymmetrical electron-density distribution.
The way in which the structures have been created also enables the researchers to perform post-functionalization via C–H borylation methods to further tune the band gap of the aGNRs. Further work is currently underway by the researchers into fine-tuning the bandgap by altering the electronic properties of the attached aryl moieties.
According to the scientists, this research has opened the door to new band-gap approaches in graphene nanoribbon research and the structures created to date already rival the band-gap transitions of donor–acceptor alternating conjugated polymers.
- A modular synthetic approach for band-gap engineering of armchair graphene nanoribbons,
Gang Li, Ki-Young Yoon, Xinjue Zhong, Jianchun Wang, Rui Zhang, Jeffrey R. Guest, Jianguo Wen, X.-Y. Zhu, Guangbin Dong,
Nature Communic. 2018.
https://doi.org/10.1038/s41467-018-03747-2