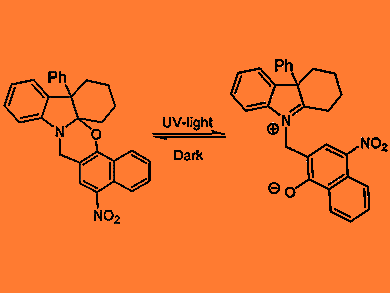
Novel photochromic compounds have been developed by chemists at the University of Coimbra and the Vila Real, Universidade de Trás-os-Montes e Alto Douro in Portugal.
Hindered photochromic benzo- and naphtho[1,3]oxazines can switch rapidly between colorless and colored forms under ultraviolet irradiation. By blasting the uncolored form with a 10 nanosecond pulse of laser light, the team can cleave the molecules’ C–O bond and opens the oxazine ring in less than 20 ns. This produces a
thermally unstable colored zwitterionic species, which reverts to the closed form, again in mere nanoseconds. The total cycle time is less than 100 ns, the team says.
Such photochromic materials could find a place in future optoelectronics devices.
- Photochromic and photophysical properties of new benzo- and naphtho[1,3]oxazine switches,
Yaroslav Prostota, Paulo Jorge Coelho, João Pina and João Seixas de Melo
Photochem. Photobiol. Sci. 2011, 10, 1346–1354.
DOI: 10.1039/C1PP05067B