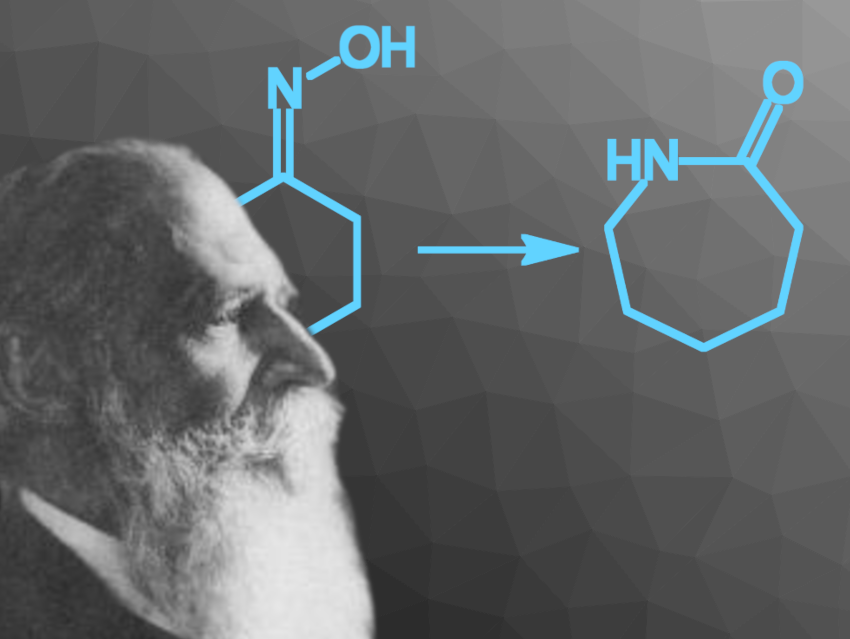
Ernst Otto Beckmann was a German pharmacist and chemist who is best known for the discovery of the Beckmann rearrangement and the development of the Beckmann differential thermometer [1–4]. He also made a large number of other contributions, e.g., the inventions of a new sodium press and an atomizer used to produce colored flames, as well as methods for the determination of molecular weights via the measurement of boiling and freezing points. He was also considered an outstanding teacher.
1 Beckmann’s Life and Career
Ernst Otto Beckmann was born on July 4, 1853, in Solingen, Germany. He performed his first chemical experiments in the laboratory of his father’s factory for dyes and pigments and became an apothecary’s apprentice at the age of 17. Bored with the work, he returned home, but his family pointed out that his ultimate plan of becoming a chemist was unrealistic if he could not complete even a simple apprenticeship in a pharmacy.
Beckmann returned to his apprenticeship and completed it, working as an apothecary’s assistant afterward. In 1874, he joined the laboratory of Carl Remigius Fresenius in Wiesbaden, Germany, where he soon became an assistant. A year later, Beckmann moved to the University of Leipzig, Germany, where his interests turned to chemistry. Nevertheless, he completed his pharmacy studies first and passed his state examination. He completed his Ph.D. in chemistry under the supervision of Hermann Kolbe and Ernst von Meyer in 1878 with work on the oxidation of organic sulfides using potassium permanganate.
Beckmann then served in the military for a year, serving as an apothecary for part of this time, before moving to the Technical University of Braunschweig, Germany in 1879 to work with the well-known toxicologist Robert Otto. He completed his habilitation there in 1882 and returned to Leipzig. However, his qualifications as a lecturer were not recognized there, and Beckmann attended classes at a secondary school to learn Greek and Latin and complete the necessary certifications, which he did in 1884, followed by a second habilitation in 1885.
Beckmann left Leipzig in 1891 to spend a year working at the University of Gießen, Germany, and then served as a Professor at the University of Erlangen, Germany, from 1892 to 1897. Then he returned to Leipzig—again—as Director of the Laboratory of Applied Chemistry. In 1912, Beckmann moved to Berlin, Germany, to head a division of the newly founded Kaiser Wilhelm Institute for Chemistry, where he remained until his retirement in 1921. Ernst Beckmann passed away on July 12, 1923.
2 Beckmann’s Research
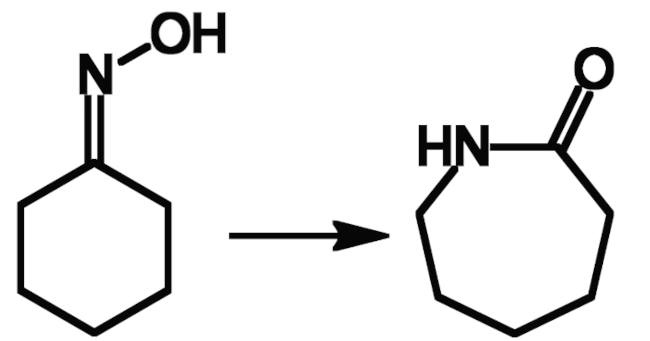
Ernst Beckmann worked on a variety of topics, spanning pharmaceutical, organic, inorganic, analytical, physical, food, and applied chemistry. His best-known discovery, the Beckmann rearrangement (a reaction that converts an oxime to a substituted amide) is still important today, e.g., for the production of ε-caprolactam (pictured below) from cyclohexanone, which is first converted to its oxime. ε-Caprolactam is used as a monomer to make Nylon fibers.
2.1 The Beckmann Rearrangement
Beckmann published his discovery of the rearrangement of oximes, later named after him, in 1886 [5]. He had found that benzophenone oxime is converted to benzanilide when treated with phosphorus pentachloride followed by alcohol and alkali.
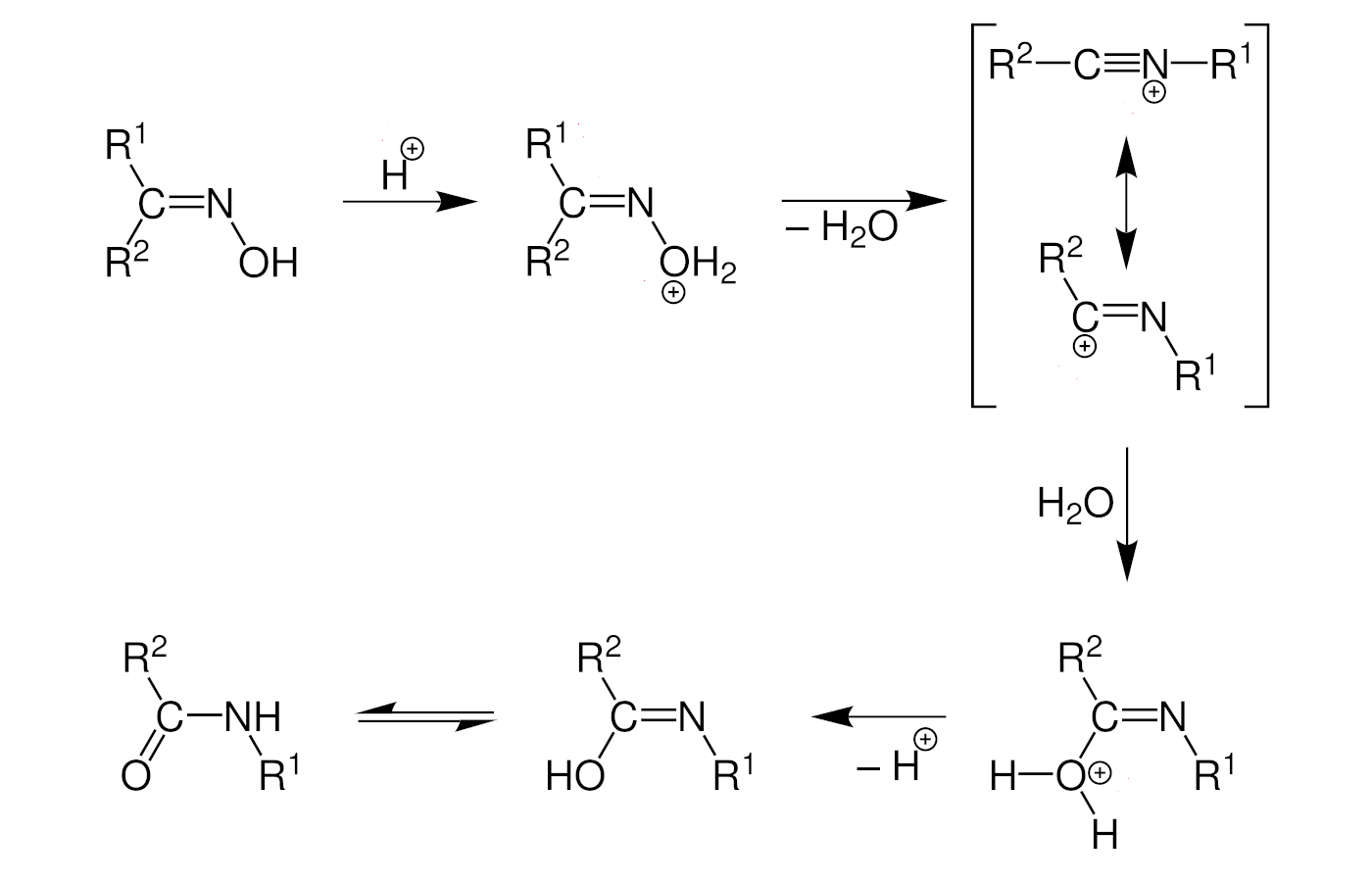
The Beckmann rearrangement is catalyzed, e.g., by an acid (mechanism pictured below). The oxime is first protonated, and water acts as a leaving group to give a nitrilium ion. An alkyl migration, followed by solvolysis and tautomerization, then give the desired amide product.
2.2 The Beckmann Differential Thermometer
 During Beckmann’s work on the determination of molecular weights, he needed exact temperature measurements, which were very difficult to conduct with existing equipment. More precisely, he measured the elevation of the boiling point and the depression of the freezing point of solutions to determine molecular masses, which requires measuring relative temperature changes rather than absolute temperatures [6,7].
During Beckmann’s work on the determination of molecular weights, he needed exact temperature measurements, which were very difficult to conduct with existing equipment. More precisely, he measured the elevation of the boiling point and the depression of the freezing point of solutions to determine molecular masses, which requires measuring relative temperature changes rather than absolute temperatures [6,7].
To achieve the required accuracy, Beckmann invented the differential thermometer (upper part with reservoir pictured on the right) that is named after him now. The thermometer’s length is usually around 40–50 cm. The accuracy of the instrument is high (ca. 0.01 °C), however, the temperature scale typically covers only about 5 °C due to the limited length of the thermometer.
The temperature range of a typical mercury thermometer is fixed. Beckmann’s design features a reservoir at the upper end of the thermometer’s tube, which is filled with mercury. The amount of mercury in the bulb can be adjusted using this mercury reservoir so that the instrument can be set to measure temperature differences at either higher or lower temperatures.
Ernst Otto Beckmann is the answer to Guess the Chemist (139).
References
[1] Obituary: Ernst Beckmann (1853–1923),
Ber. Dtsch. Chem. Ges. 1928, 61, A87–A130.
https://doi.org/10.1002/cber.19280610728
[2] Ernst Beckmann, 1853–1923,
R. E. Oesper,
J. Chem. Educ. 1944, 21, 10, 470–475.
https://doi.org/10.1021/ed021p470
[3] Beckmann, Ernst,
G. Lockemann,
in Neue Deutsche Biographie 1953, 1, 725–726.
[4] Ernst Beckmann,
U. Messow,
in Sächsische Biografie 2010.
[5] Zur Kenntniss der Isonitrosoverbindungen,
E. Beckmann,
Ber. Dtsch. Chem. Ges. 1886, 19, 988–993.
https://doi.org/10.1002/cber.188601901222
[6] Bestimmung des Molekulargewichts aus Siedepunktserhöhungen,
E. Beckmann,
Z. Phys. Chem. 1889, 3, 603–604.
[7] Studien zur Praxis der Bestimmung des Molekulargewichts aus Dampfdruckerniedrigungen,
E.Beckmann,
Z. Phys. Chem. 1889, 4, 532–552.
Also of Interest
Video: Great Architecture and Chemists in Dahlem,
ChemistryViews 2018.
Walking tour through Dahlem, one of the richest parts of Berlin and an important historical center for academic research