Johannes Diderik van der Waals was born on November 23, 1837, in Leiden in The Netherlands. He was a theoretical physicist and thermodynamicist known for his pioneering work on the equation of state for gases and liquids. His name also appears in concepts such as Van der Waals forces and Van der Waals radii. He won the Nobel Prize in Physics in 1910 “for his work on the equation of state for gases and liquids” [1].
Van der Waals is most closely associated with the Van der Waals equation of state, which describes the behavior of gases and their condensation to the liquid phase. He also accounted for the finite size of the molecules. Van der Waals was able to obtain estimates for the actual size of molecules and the strength of their mutual attraction by comparing his equation of state with experimental data, which revolutionized the study of equations of state. His work had a direct and fundamental effect on molecular physics in the 20th century. Van der Waals started his career as a school teacher before becoming the first Professor of Physics at the University of Amsterdam.
1 Becoming a Professor without a Classical Education
Johannes Diderik van der Waals was born on November 23, 1837, in Leiden, The Netherlands. After finishing school, he worked as a teacher in his hometown. He wanted to pursue university studies but lacked training in classical languages, which was a requirement at the time. Nevertheless, from 1862 to 1865, van der Waals attended lectures and seminars in physics and mathematics at the University of Leiden in his free time. This enabled him to expand his teaching qualifications. From 1864 on, he worked as a teacher in Deventer, Netherlands, and in 1866, he took up a post at a secondary school in The Hague, Netherlands, where he later became principal.
A change in the law eliminating the language requirements allowed van der Waals to study natural sciences. In 1873, he graduated with a dissertation in physics that described the critical point of a gas [2], laying the foundation for his future work on the equation of state for gases and liquids. From 1877 to 1908, he served as Professor of Physics at the newly founded municipal University of Amsterdam, The Netherlands.
Johannes Diderik van der Waals passed away in Amsterdam on March 8, 1923.
2 Research
2.1 The Van der Waals Equation of State
The ideal gas law is pV = nRT. It describes the behavior of an idealized gas under different conditions. p stands for the pressure, V for the volume, n for the number of particles, R for the ideal gas constant, and T for the temperature. However, this equation of state neglects intermolecular interactions between the molecules in a gas, as well as the finite sizes of the molecules [3].
The Van der Waals equation of state includes the effects of the intermolecular interactions and non-zero molecular sizes. It describes experimentally observed processes that the ideal gas law cannot account for. The first derivations of this equation appeared in his dissertation [2].
Molecular Sizes
To account for the molecular sizes, the Van der Waals equation replaces the term V/n in the ideal gas law with (Vm–b), where Vm stands for the molar volume of the gas and b is called the “excluded volume” or “co-volume”, which is roughly four times the total molecular volume for one mole of the gas. This accounts for the fact that two molecules cannot occupy the same space.
Intermolecular Interactions
To include the effects of intermolecular interactions, the term a/Vm2 is added to the term for the pressure. Again, Vm is the molar volume, while a is a constant that depends on the gas and reflects the average attraction between its particles.
The overall van der Waals equation is, thus,
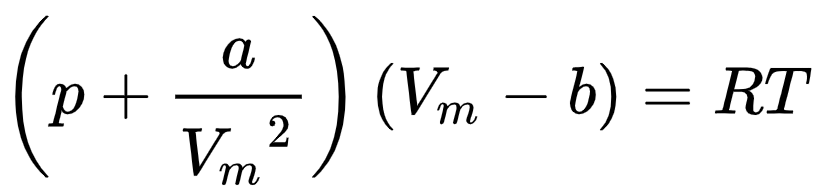
2.2 The Principle of Corresponding States
The constants a and b in the van der Waals equation depend on the specific compound. The equation can, however, also be rewritten in a reduced form that applies to all compounds. For this one defines reduced variables that depend on the critical pressure pcrit, the critical temperature Tcrit, and the critical molar volume vcrit. The reduced pressure pR, for example, then is pR = p/pcrit.
With these reduced variables, the van der Waals equation can be rewritten as
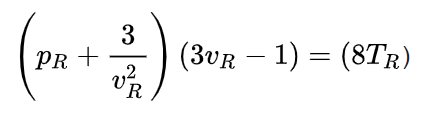
In this form, the equation applies to all gases. This is an example of the principle of corresponding states proposed by van der Waals. It states that all fluids (liquids and gases), when compared at the same reduced temperature and reduced pressure, have approximately the same compressibility factor, i.e., they deviate from ideal gas behavior to about the same degree. Van der Waals’s reduced equation predicts a compressibility factor Zcrit at the critical point of Zcrit = (pcritvcrit)/(RTcrit) = 3/8 . In reality, the compressibility factor differs for different gases.
2.3 Van der Waals Forces
Van der Waals forces are distance-dependent interactions between atoms or molecules. They do not result from a chemical bond and are comparatively weaker than ionic or covalent bonds. They are named after van der Waals because he was one of the first to postulate such an intermolecular force.
Today, van der Waals forces are considered a combination of effects such as the London dispersion force between fluctuating and induced dipoles, the Debye force between permanent dipoles and induced dipoles, and the Keesom force between permanent dipoles.
2.4 Van der Waals Radii
The van der Waals radius of an atom is the radius of an imaginary hard sphere that serves as a model for an atom. It is calculated, e.g., from the distances of pairs of unbonded atoms in crystals. Similar to the van der Waals forces, it is named after van der Waals because he was one of the first to recognize that atoms have a non-zero size, which has consequences as shown in the van der Waals equation of state.
References
[1] Johannes Diderik van der Waals – Facts, NobelPrize.org. (accessed February 23, 2023)
[2] Johannes Diderik van der Waals, Over de Continuiteit van den Gas- en Vloeistoftoestand, Dissertation, Universität Leiden, Netherlands, 1873.
[3] Peter W. Atkins, Julio de Paula, Physikalische Chemie, Wiley-VCH, Weinheim, 2013. ISBN: 978-3-527-33247-2





Es un legado un eminente físico, deja una huella para la ciencia e historia de la humanidad