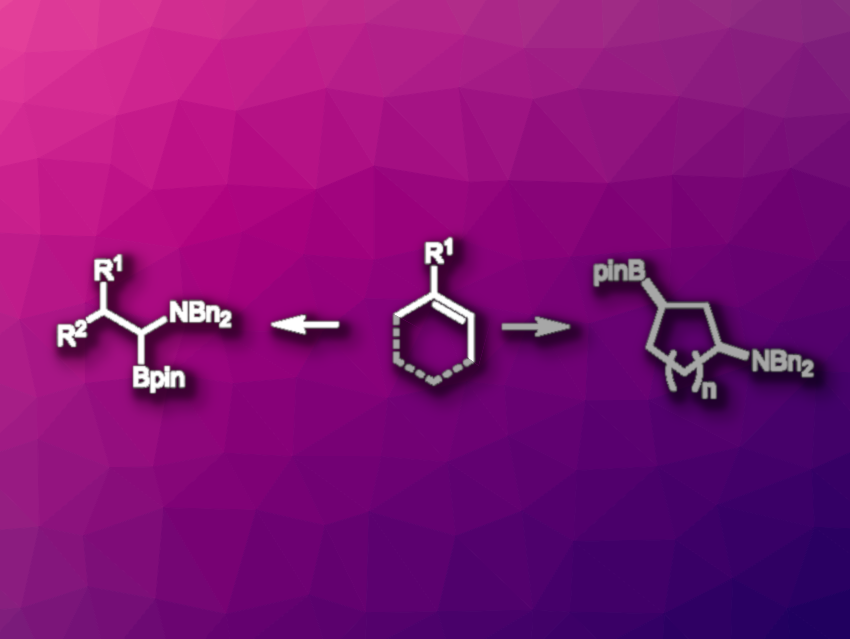
Alkene functionalizations are useful transformations in organic synthesis that allow the construction of complex molecules. Alkene borylaminations, for example, introduce a nitrogen functionality and a C–B bond that is useful for further functionalization at the same time. 1,2-Borylamination reactions are known for both activated and unactivated alkenes, while the related 1,1- and 1,3-borylaminations have remained challenging.
Shuming Chen, Oberlin College, OH, USA, M. Kevin Brown, Indiana University, Bloomington, USA, and colleagues have developed a method for the nickel-catalyzed 1,1-borylamination of 1,1-disubstituted and monosubstituted alkenes, which was extended to the 1,3-borylamination of cyclic alkenes (general reactions pictured above). The team used NiCl2·DME (DME = dimethoxyethane ) as the catalyst, (Bpin)2 (bis(pinacolato)diboron) as the borylation reagent together with t-BuLi as an activating agent, MgBr2–OEt2 as an additive, and a mixture of tetrahydrofuran (THF) and dimethylacetamide (DMA) as the solvent.
Under these conditions, the researchers reacted a variety of 1,1-disubstituted and monosubstituted alkenes with amines such as Bn2NOBz. The desired 1,1-aminoboration products were obtained in moderate yields, and the reaction was also performed on a gram scale. The team then tested the reaction with cyclic alkenes and found that a 1,3-aminoboration occurs (pictured above in grey). Overall, the developed reaction is useful for the synthesis of versatile difunctionalized products.
- Ni-Catalyzed 1,1- and 1,3-Aminoboration of Unactivated Alkenes,
Mao-Yun Lyu, Gabriel N. Morais, Shuming Chen, M. Kevin Brown,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c12770