Small carbocycles often have interesting reactivities due to their ring strain. Replacing carbon atoms with boron-based units can increase the ring strain even further. Thus, three- and four-membered boracarbocycles tend to be reactive and can be challenging to study. Dihydrodiboretes, for example, are four-membered C2B2 heterocycles that can be considered isoelectronic to the cyclobutadiene dication ([C4H4]2+). 1,2-Dihydro-1,2-diboretes tend to be unstable and rearrange into more stable isomers. Sterically demanding substituents and fusing the ring to an arene ring can help to stabilize such species.
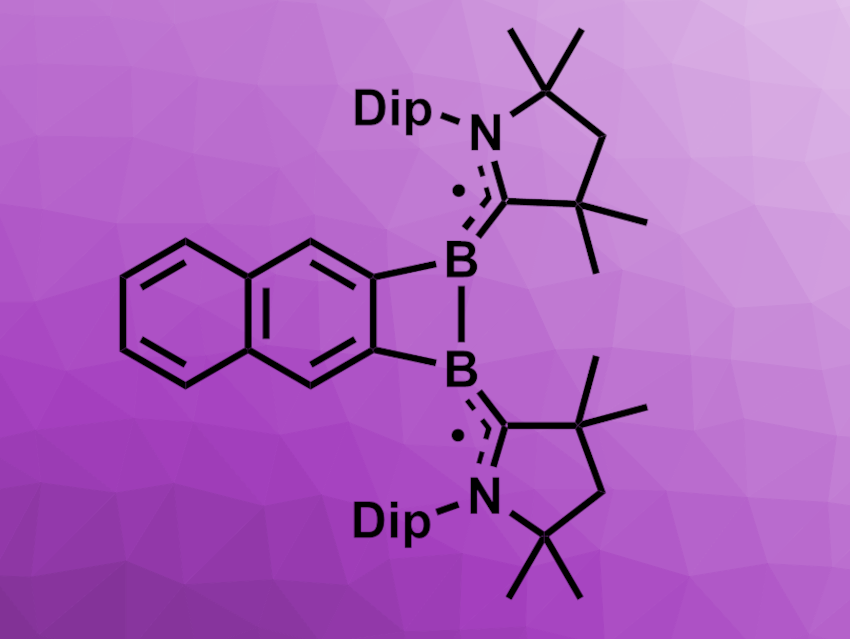
Felipe Fantuzzi, University of Kent, Canterbury, UK, Holger Braunschweig, University of Würzburg, Germany, and colleagues have synthesized a highly strained arene-fused 1,2-diborete biradicaloid stabilized by cyclic alkyl(amino)carbene (CAAC) units (pictured). The team started from 2,3-bis(dibromoboryl)naphthalene, which was reacted with the CAAC 1-(2,6-diisopropylphenyl)-3,3,5,5-tetramethylpyrrolidin-2-ylidene to obtain a bis(CAAC) adduct. This adduct can be reduced using KC8 to obtain mono(bromoboryl) radical or bis(bromoboryl) biradical species.
When the bis(CAAC) adduct is reduced using lithium sand in tetrahydrofuran (THF) at room temperature, a CAAC-stabilized 1,2-diborete (pictured) is obtained as a green solid in a yield of 41 %. The results of NMR and solid-state EPR spectroscopy indicate that the product has a biradical character. X-ray crystallography confirmed the formation of a four-membered 1,2-diborete ring.
- Highly Strained Arene-Fused 1,2-Diborete Biradicaloid,
Annalena Gärtner, Lukas Meier, Merle Arrowsmith, Maximilian Dietz, Ivo Krummenacher, Rüdiger Bertermann, Felipe Fantuzzi, Holger Braunschweig,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c09971