Georg Wittig was born on June 16, 1897, in Berlin, Germany. He grew up in Kassel, Germany, and started to study chemistry at the University of Tübingen, Germany. His studies were interrupted by World War I, and after some difficulties finding a place at a university post-war, he continued them in Marburg, Germany, working under Karl von Auwers. He completed his Ph.D. there in 1923 and his habilitation in 1926. During his time in Marburg, Wittig became friends with Karl Ziegler, who would go on to win the Nobel Prize in Chemistry in 1963 (together with Giulio Natta) for his work on polymers.
After working as a Lecturer at the University of Marburg from 1926 to 1932, Wittig became Adjunct Professor in Braunschweig, Germany, where he stayed until 1937. Then he served as Associate Professor in Freiburg, Germany, and in 1944, he moved to Tübingen to serve as Full Professor of Organic Chemistry. In 1956, Wittig moved to the University of Heidelberg, Germany, where he served as Head of the Organic Chemistry Department and remained until his retirement in 1967. Georg Wittig died on August 26, 1987, in Heidelberg.
Awards
Georg Wittig is best known for the discovery of the Wittig olefination (see below) and received the 1979 Nobel Prize for Chemistry for this breakthrough work in organic synthesis (together with Herbert C. Brown, who was honored for his work on organoboranes).
Among many other honors, he also received the Adolf von Baeyer Memorial Medal from the German Chemical Society (GDCh) in 1953, the Otto Hahn Prize for Chemistry and Physics in 1967, and the Paul Karrer Gold Medal from the University of Zurich, Switzerland, in 1972. Wittig was a Member of the Bavarian Academy of Sciences and Humanities, the Heidelberg Academy of Sciences and Humanities, the German National Academy of Sciences Leopoldina, and the French Academy of Sciences.
Research
Among other topics, Georg Wittig worked on organic radicals, used phenyllithium for the metalation of organic compounds, developed the eponymous Wittig reaction, and discovered the [1,2]-Wittig rearrangement. Examples are shown below.
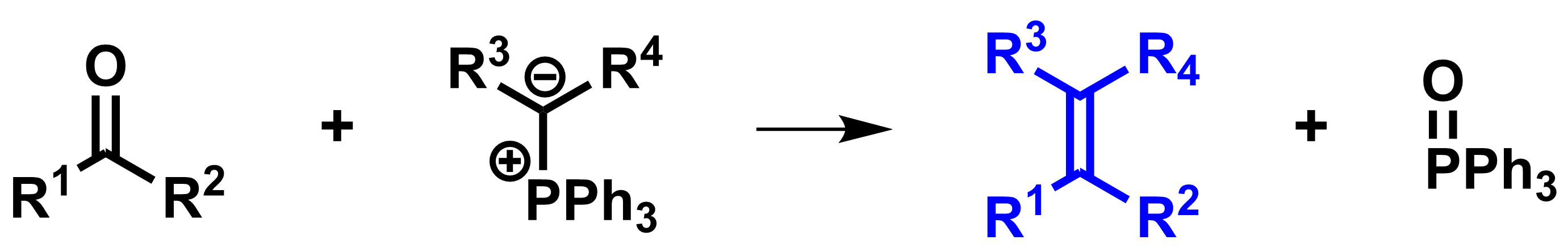
Wittig Reaction
The Wittig reaction [1,2], or Wittig olefination, is a reaction of an aldehyde or ketone with a phosphonium ylide, or Wittig reagent. In this transformation (pictured below), the carbonyl is converted to an alkene. The reaction is driven by the formation of a strong P=O bond in the phosphine oxide byproduct (most often Ph3P=O). The reaction is widely used in organic synthesis—often to introduce a methylene group (=CH2).
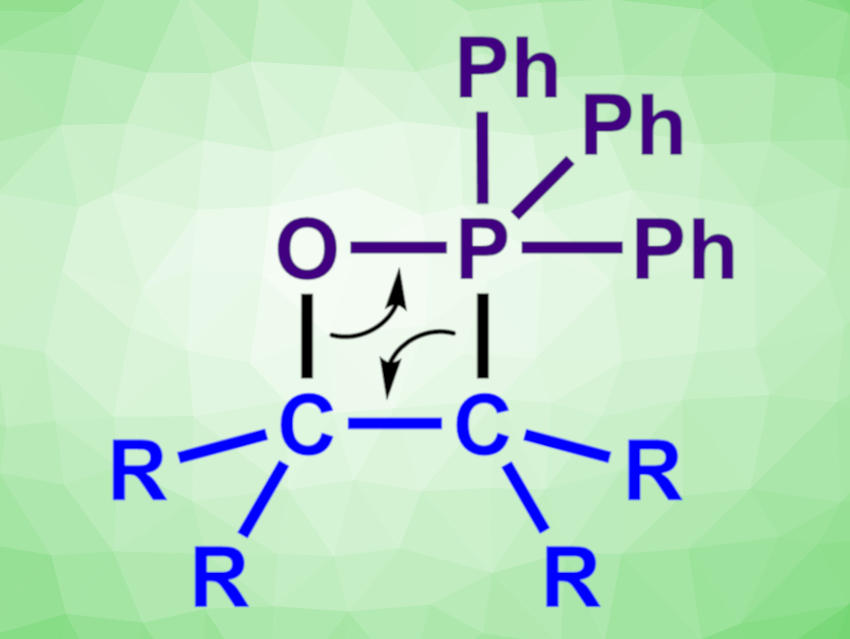
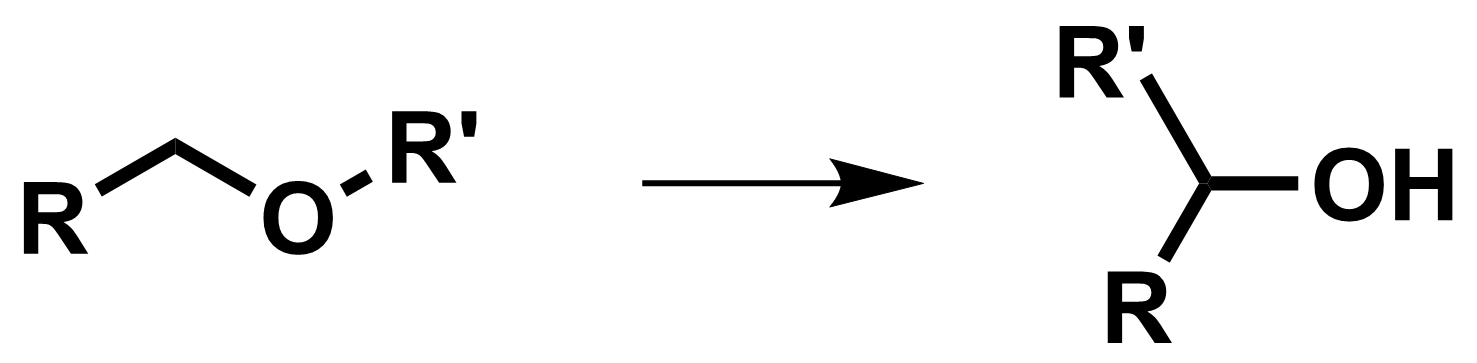
[1,2]-Wittig Rearrangement
The [1,2]-Wittig rearrangement is a base-promoted reaction of ethers to give alcohols [3,4].
Georg Wittig is the answer to Guess the Chemist (126).
References
- [1] Über Triphenyl‐phosphin‐methylene als olefinbildende Reagenzien (I. Mitteil.),
G. Wittig, U. Schöllkopf,
Chem. Ber. 1954, 87, 1318–1330.
https://doi.org/10.1002/cber.19540870919 - [2] Über Triphenyl‐phosphinmethylene als olefinbildende Reagenzien (II. Mitteil.),
G. Wittig, W. Haag,
Chem. Ber. 1955, 88, 1654–1666.
https://doi.org/10.1002/cber.19550881110 - [3] Über die kationotrope Isomerisation gewisser Benzyläther bei Einwirkung von Phenyl-lithium,
G. Wittig, L. Löhmann,
Liebig’s Ann. 1942, 550, 260–268.
https://doi.org/10.1002/jlac.19425500117 - [4] Ergebnisse und Probleme der organischen Anionochemie,
G. Wittig,
Experientia 1958, 14, 389–395.
https://doi.org/10.1007/BF02160421
Sources
- Georg Wittig (1897–1987),
W. Tochtermann,
Liebigs Ann. 1997, I–XXI.
https://doi.org/10.1002/jlac.199719970303 - Wittig and His Accomplishments: Still Relevant Beyond His 100th Birthday,
R. W. Hoffmann,
Angew. Chem. Int. Ed. 2001, 40, 1411–1416.
https://doi.org/10.1002/1521-3773(20010417)40:8<1411::AID-ANIE1411>3.0.CO;2-U